Epigenetic DNA modifications and vitamin C in prostate cancer and benign prostatic hyperplasia: Exploring similarities, disparities, and pathogenic implications
- PMID: 39471555
- PMCID: PMC11550371
- DOI: 10.1016/j.neo.2024.101079
Epigenetic DNA modifications and vitamin C in prostate cancer and benign prostatic hyperplasia: Exploring similarities, disparities, and pathogenic implications
Abstract
Objectives: Benign Prostatic Hyperplasia (BPH) and Prostate Cancer (PC) are very common pathologies among aging men. Both disorders involve aberrant cell division and differentiation within the prostate gland. However, the direct link between these two disorders still remains controversial. A plethora of works have demonstrated that inflammation is a major causative factor in both pathologies. Another key factor involved in PC development is DNA methylation and hydroxymethylation.
Methods: A broad spectrum of parameters, including epigenetic DNA modifications and 8-oxo-7,8-dihydro-2'-deoxyguanosine, was analyzed by two-dimensional ultraperformance liquid chromatography with tandem mass spectrometry in tissues of BPH, PC, and marginal one, as well as in leukocytes of the patients and the control group. In the same material, the expression of TETs and TDG genes was measured using RT-qPCR. Additionally, vitamin C was quantified in the blood plasma and within cells (leukocytes and prostate tissues).
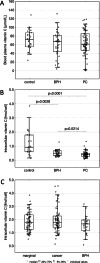
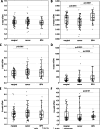
Results: Unique patterns of DNA modifications and intracellular vitamin C (iVC) in BPH and PC tissues, as well as in leukocytes, were found in comparison with control samples. The majority of the alterations were more pronounced in leukocytes than in the prostate tissues.
Conclusions: Characteristic DNA methylation/hydroxymethylation and iVC profiles have been observed in both PC and BPH, suggesting potential shared molecular pathways between the two conditions. As a fraction of leukocytes may be recruited to the pathological tissues and can migrate back into the circulation/blood, the observed alterations in leukocytes may reflect dynamic changes associated with the PC development, suggesting their potential utility as early markers of prostate cancer development.
Keywords: Benign prostatic hyperplasia; Epigenetic DNA modifications; Prostate cancer; TET enzymes; Vitamin C.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Hudson D.L., Guy A.T., Fry P., O'Hare M.J., Watt F.M., Masters J.R. Epithelial cell differentiation pathways in the human prostate: identification of intermediate phenotypes by keratin expression. J. Histochem. Cytochem. 2001;49:271–278. - PubMed
-
- Orsted D.D., Bojesen S.E. The link between benign prostatic hyperplasia and prostate cancer. Nat. Rev. Urol. 2013;10:49–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

