ARP2/3 complex affects myofibroblast differentiation and migration in pancreatic ductal adenocarcinoma
- PMID: 39472297
- PMCID: PMC11737003
- DOI: 10.1002/ijc.35246
ARP2/3 complex affects myofibroblast differentiation and migration in pancreatic ductal adenocarcinoma
Abstract
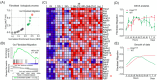
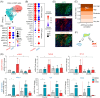
The ARP2/3 complex, which orchestrates actin cytoskeleton organization and lamellipodia formation, has been implicated in the initiation of pancreatic ductal adenocarcinoma (PDAC). This study aims to clarify its impact on the activity of cancer-associated fibroblasts (CAFs), key players in PDAC progression, and patient outcomes. Early pancreatic carcinogenesis was modeled in p48Cre; LSL-KrasG12D mice with caerulein-induced pancreatitis, complemented by in vitro studies on human immortalized pancreatic stellate cells (PSCs) and primary PDAC-derived CAFs. Data were gained from microarray analysis, RNA sequencing (RNA-seq), and single-cell RNA sequencing (sc-RNA-seq), with subsequent bioinformatics analysis. We uncovered a specific transcriptional signature associated with fibroblast migration in early pancreatic carcinogenesis and linked it to poor survival in patients with PDAC. A pivotal role of the ARP2/3 complex in CAF migration was identified. Inhibition of the ARP2/3 complex markedly decreased CAF motility and induced significant morphological changes in vitro. Furthermore, its inhibition also hindered TGFβ1-mediated myofibroblastic CAF differentiation but had no effect on IL-1-mediated inflammatory CAF differentiation. Our findings position the ARP2/3 complex as central to the migration and differentiation of myofibroblastic CAF. Targeting this complex presents a promising new therapeutic avenue for PDAC treatment.
Keywords: cancer‐associated fibroblasts; myCAFs; pancreatic carcinogenesis; transcriptional signature; transforming growth factor β.
© 2024 The Author(s). International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Chandana S, Babiker HM, Mahadevan D. Therapeutic trends in pancreatic ductal adenocarcinoma (PDAC). Expert Opin Investig Drugs. 2019;28:161‐177. - PubMed
-
- Costa A, Kieffer Y, Scholer‐Dahirel A, et al. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33(463–79):e10. - PubMed
-
- Lambrechts D, Wauters E, Boeckx B, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med. 2018;24:1277‐1289. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

