Eustigmatophyte model of red-shifted chlorophyll a absorption in light-harvesting complexes
- PMID: 39472488
- PMCID: PMC11522437
- DOI: 10.1038/s42003-024-07101-9
Eustigmatophyte model of red-shifted chlorophyll a absorption in light-harvesting complexes
Abstract
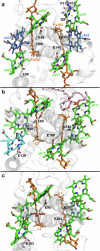
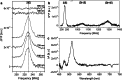
Photosynthetic organisms harvest light for energy. Some eukaryotic algae have specialized in harvesting far-red light by tuning chlorophyll a absorption through a mechanism still to be elucidated. Here, we combined optically detected magnetic resonance and pulsed electron paramagnetic resonance measurements on red-adapted light-harvesting complexes, rVCP, isolated from the freshwater eustigmatophyte alga Trachydiscus minutus to identify the location of the pigments responsible for this remarkable adaptation. The pigments have been found to belong to an excitonic cluster of chlorophylls a at the core of the complex, close to the central carotenoids in L1/L2 sites. A pair of structural features of the Chl a403/a603 binding site, namely the histidine-to-asparagine substitution in the magnesium-ligation residue and the small size of the amino acid at the i-4 position, resulting in a [A/G]xxxN motif, are proposed to be the origin of this trait. Phylogenetic analysis of various eukaryotic red antennae identified several potential LHCs that could share this tuning mechanism. This knowledge of the red light acclimation mechanism in algae is a step towards rational design of algal strains in order to enhance light capture and efficiency in large-scale biotechnology applications.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Red-shifted light-harvesting system of freshwater eukaryotic alga Trachydiscus minutus (Eustigmatophyta, Stramenopila).Photosynth Res. 2019 Nov;142(2):137-151. doi: 10.1007/s11120-019-00662-5. Epub 2019 Aug 2. Photosynth Res. 2019. PMID: 31375979
-
Energy transfer dynamics in a red-shifted violaxanthin-chlorophyll a light-harvesting complex.Biochim Biophys Acta Bioenerg. 2019 Feb 1;1860(2):111-120. doi: 10.1016/j.bbabio.2018.11.006. Epub 2018 Nov 8. Biochim Biophys Acta Bioenerg. 2019. PMID: 30414929
-
The nature of a chlorophyll ligand in Lhca proteins determines the far red fluorescence emission typical of photosystem I.J Biol Chem. 2003 Dec 5;278(49):49223-9. doi: 10.1074/jbc.M309203200. Epub 2003 Sep 22. J Biol Chem. 2003. PMID: 14504274
-
Far-red light acclimation in diverse oxygenic photosynthetic organisms.Photosynth Res. 2019 Dec;142(3):349-359. doi: 10.1007/s11120-019-00653-6. Epub 2019 Jun 19. Photosynth Res. 2019. PMID: 31222688 Review.
-
Light harvesting complexes in chlorophyll c-containing algae.Biochim Biophys Acta Bioenerg. 2020 Apr 1;1861(4):148027. doi: 10.1016/j.bbabio.2019.05.003. Epub 2019 May 31. Biochim Biophys Acta Bioenerg. 2020. PMID: 31153887 Review.
Cited by
-
Far-Red Absorbing LHCII Incorporating Chlorophyll d Preserves Photoprotective Carotenoid Triplet-Triplet Energy Transfer Pathways.J Phys Chem Lett. 2025 Feb 20;16(7):1720-1728. doi: 10.1021/acs.jpclett.4c03463. Epub 2025 Feb 10. J Phys Chem Lett. 2025. PMID: 39928962 Free PMC article.
-
Kinetics of Chlorophyll Degradation in Japanese Maple (Acer palmatum) Leaves with In Situ Heating Visible and Near-Infrared Spectroscopic Monitoring.Life (Basel). 2025 Feb 21;15(3):335. doi: 10.3390/life15030335. Life (Basel). 2025. PMID: 40141681 Free PMC article.
References
-
- Green, B. R. & Kühlbrandt, W. Sequence conservation of light-harvesting and stress-response proteins in relation to the three-dimensional molecular structure of LHCII. Photosynth. Res.44, 139–148 (1995). - PubMed
-
- Liu, Z. et al. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature428, 287–292 (2004). - PubMed
-
- Renger, T., Madjet, M. E., Knorr, A. & Müh, F. How the molecular structure determines the flow of excitation energy in plant light-harvesting complex II. J. Plant Physiol.168, 1497–1509 (2011). - PubMed
-
- Novoderezhkin, V., Marin, A. & van Grondelle, R. Intra- and inter-monomeric transfers in the light harvesting LHCII complex: the Redfield–Förster picture. Phys. Chem. Chem. Phys.13, 17093 (2011). - PubMed
-
- Carbonera, D. et al. Photoprotective sites in the violaxanthin–chlorophyll a binding Protein (VCP) from Nannochloropsis gaditana. Biochim. Biophys. Acta - Bioenerg.1837, 1235–1246 (2014). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

