Eustigmatophyte model of red-shifted chlorophyll a absorption in light-harvesting complexes
- PMID: 39472488
- PMCID: PMC11522437
- DOI: 10.1038/s42003-024-07101-9
Eustigmatophyte model of red-shifted chlorophyll a absorption in light-harvesting complexes
Abstract
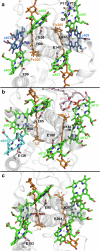
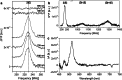
Photosynthetic organisms harvest light for energy. Some eukaryotic algae have specialized in harvesting far-red light by tuning chlorophyll a absorption through a mechanism still to be elucidated. Here, we combined optically detected magnetic resonance and pulsed electron paramagnetic resonance measurements on red-adapted light-harvesting complexes, rVCP, isolated from the freshwater eustigmatophyte alga Trachydiscus minutus to identify the location of the pigments responsible for this remarkable adaptation. The pigments have been found to belong to an excitonic cluster of chlorophylls a at the core of the complex, close to the central carotenoids in L1/L2 sites. A pair of structural features of the Chl a403/a603 binding site, namely the histidine-to-asparagine substitution in the magnesium-ligation residue and the small size of the amino acid at the i-4 position, resulting in a [A/G]xxxN motif, are proposed to be the origin of this trait. Phylogenetic analysis of various eukaryotic red antennae identified several potential LHCs that could share this tuning mechanism. This knowledge of the red light acclimation mechanism in algae is a step towards rational design of algal strains in order to enhance light capture and efficiency in large-scale biotechnology applications.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Green, B. R. & Kühlbrandt, W. Sequence conservation of light-harvesting and stress-response proteins in relation to the three-dimensional molecular structure of LHCII. Photosynth. Res.44, 139–148 (1995). - PubMed
-
- Liu, Z. et al. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature428, 287–292 (2004). - PubMed
-
- Renger, T., Madjet, M. E., Knorr, A. & Müh, F. How the molecular structure determines the flow of excitation energy in plant light-harvesting complex II. J. Plant Physiol.168, 1497–1509 (2011). - PubMed
-
- Novoderezhkin, V., Marin, A. & van Grondelle, R. Intra- and inter-monomeric transfers in the light harvesting LHCII complex: the Redfield–Förster picture. Phys. Chem. Chem. Phys.13, 17093 (2011). - PubMed
-
- Carbonera, D. et al. Photoprotective sites in the violaxanthin–chlorophyll a binding Protein (VCP) from Nannochloropsis gaditana. Biochim. Biophys. Acta - Bioenerg.1837, 1235–1246 (2014). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

