Tracing the path of Quorum sensing molecules in cystic fibrosis mucus in a biomimetic in vitro permeability platform
- PMID: 39472521
- PMCID: PMC11522324
- DOI: 10.1038/s41598-024-77375-w
Tracing the path of Quorum sensing molecules in cystic fibrosis mucus in a biomimetic in vitro permeability platform
Abstract
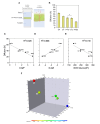
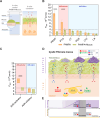
P. aeruginosa employs specific quorum sensing (QS) mechanisms to orchestrate biofilm formation, enhancing resistance to host defences. In physiological conditions, QS molecules permeate the lung environment and cellular membrane to reach the cytoplasmic Aryl Hydrocarbon Receptor (AhR) that is pivotal for activating the immune response against infection. In pathological conditions like cystic fibrosis (CF) this interkingdom communication is altered, favouring P. aeruginosa persistence and chronic infection. Here, we aim to investigate the molecular journey of QS molecules from CF-like environments to the cytoplasm by quantifying via HPLC-MS the permeability of selected QS molecules (quinolones, lactones, and phenazines) through in vitro models of the two main biological lung barriers: CF-mucus and cellular membrane. While QS molecules not activating AhR exhibit intermediate permeability through the cellular membrane model (PAMPA) (1.0-4.0 × 10-6 cm/s), the AhR-activating molecule (pyocyanin) shows significantly higher permeability (8.6 ± 1.4 × 10-6 cm/s). Importantly, combining the CF mucus model with PAMPA induces a 50% decrease in pyocyanin permeability, indicating a strong mucus-shielding effect with pathological implications in infection eradication. This study underscores the importance of quantitatively describing the AhR-active bacterial molecules, even in vitro, to offer new perspectives for understanding P. aeruginosa virulence mechanisms and for proposing new antibacterial therapeutic approaches.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Li, Z. et al. Infection and lung disease progression in children with cystic fibrosis. 293, 581–588 (2017). - PubMed
-
- Murray, M. P., Pentland, J. L., Turnbull, K., MacQuarrie, S. & Hill, A. T. Sputum colour: a useful clinical tool in non-cystic fibrosis bronchiectasis. Eur. Respir. J.34, 361–364 (2008). - PubMed
-
- Burns, J. L. et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis.183, 444–452 (2001). - PubMed
-
- Murray, T. S., Egan, M. & Kazmierczak, B. I. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr. Opin. Pediatr.19, 83–88 (2007). - PubMed
-
- Ramsey, A. K. A. et al. Page 1 of 38 1–38 (2014).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

