Neuronal excitation-inhibition imbalance in the basolateral amygdala is involved in propofol-mediated enhancement of fear memory
- PMID: 39472670
- PMCID: PMC11522401
- DOI: 10.1038/s42003-024-07105-5
Neuronal excitation-inhibition imbalance in the basolateral amygdala is involved in propofol-mediated enhancement of fear memory
Abstract
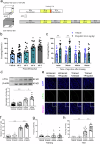
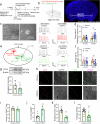
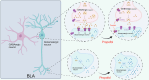
Posttraumatic stress disorder (PTSD) is associated with glutamatergic neuron hyperactivation in the basolateral amygdala (BLA) brain area, while GABAergic interneurons in the BLA modulate glutamatergic neuron excitability. Studies have shown that propofol exerts its effects through potentiation of the inhibitory neurotransmitter γ-aminobutyric acid. The neuronal mechanism by which propofol anesthesia modulates fear memory is currently unknown. Here, we used optogenetics and chemogenetics to suppress glutamatergic neurons or activate GABAergic interneurons in the BLA to assess alterations in neuronal excitation-inhibition balance and investigate fear memory. The excitability of glutamatergic neurons in the BLA was significantly reduced by the suppression of glutamatergic neurons or activation of GABAergic interneurons, while propofol-mediated enhancement of fear memory was attenuated. We suggest that propofol anesthesia could reduce the excitability of GABAergic neurons through activation of GABAA receptors, subsequently increasing the excitability of glutamatergic neurons in the mice BLA; the effect of propofol on enhancing mice fear memory might be mediated by strengthening glutamatergic neuronal excitability and decreasing the excitability of GABAergic neurons in the BLA; neuronal excitation-inhibition imbalance in the BLA might be important in mediating the enhancement of fear memory induced by propofol.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

