State of the art of mobile health technologies use in clinical arrhythmia care
- PMID: 39472742
- PMCID: PMC11522556
- DOI: 10.1038/s43856-024-00618-4
State of the art of mobile health technologies use in clinical arrhythmia care
Abstract
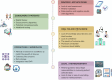
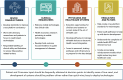
The rapid growth in consumer-facing mobile and sensor technologies has created tremendous opportunities for patient-driven personalized health management. The diagnosis and management of cardiac arrhythmias are particularly well suited to benefit from these easily accessible consumer health technologies. In particular, smartphone-based and wrist-worn wearable electrocardiogram (ECG) and photoplethysmography (PPG) technology can facilitate relatively inexpensive, long-term rhythm monitoring. Here we review the practical utility of the currently available and emerging mobile health technologies relevant to cardiac arrhythmia care. We discuss the applications of these tools, which vary with respect to diagnostic performance, target populations, and indications. We also highlight that requirements for successful integration into clinical practice require adaptations to regulatory approval, data management, electronic medical record integration, quality oversight, and efforts to minimize the additional burden to health care professionals.
© 2024. The Author(s).
Conflict of interest statement
A.R.S.: an Editorial Board member for Communications Medicine and Guest Editor for the Cardiac Arrhythmias Collection, but was not involved in the editorial review or peer review, nor the decision to publish this article. D.L.: consultant to Phillips Medical and Johnson & Johnson. K.T.: employed with Medtronic. M.T.: employed with IRhythm Technologies. Y.F.: employed with Cardiologs.
Figures




References
-
- Bethge, K. P. Classification of arrhythmias. J. Cardiovasc. Pharm.17, S13–S19 (1991). - PubMed
-
- Pahlm, O. & Sórnmo, L. Software QRS detection in ambulatory monitoring—a review. Med. Biol. Eng. Comput.22, 289–297 (1984). - PubMed
-
- Sanders, D., Ungar, L., Eskander, M. A. & Seto, A. H. Ambulatory ECG monitoring in the age of smartphones. Cleve Clin. J. Med.86, 483–493 (2019). - PubMed
-
- SmartCardia. Smartcardia 7L Patch Monitor.
Publication types
LinkOut - more resources
Full Text Sources

