Specific ECM degradation potentiates the antitumor activity of CAR-T cells in solid tumors
- PMID: 39472748
- PMCID: PMC11606952
- DOI: 10.1038/s41423-024-01228-9
Specific ECM degradation potentiates the antitumor activity of CAR-T cells in solid tumors
Abstract
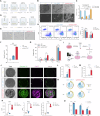
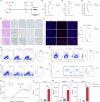
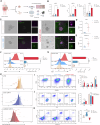
Although major progress has been made in the use of chimeric antigen receptor (CAR)-T-cell therapy for hematological malignancies, this method is ineffective against solid tumors largely because of the limited infiltration, activation and proliferation of CAR-T cells. To overcome this issue, we engineered CAR-T cells with synthetic Notch (synNotch) receptors, which induce local tumor-specific secretion of extracellular matrix (ECM)-degrading enzymes at the tumor site. SynNotch CAR-T cells achieve precise ECM recognition and robustly kill targeted tumors, with synNotch-induced enzyme production enabling the degradation of components of the tumor ECM. In addition, this regulation strongly increased the infiltration of CAR-T cells and the clearance of solid tumors, resulting in tumor regression without toxicity in vivo. Notably, synNotch CAR-T cells also promoted the persistent activation of CAR-T cells in patient-derived tumor organoids. Thus, we constructed a synthetic T-cell system that increases the infiltration and antitumor function of CAR-T cells, providing a strategy for targeting ECM-rich solid tumors.
Keywords: CAR-T cells; ECM-degrading enzymes; Infiltration; synNotch receptor.
© 2024. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
Competing interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures



References
-
- Maus MV. A decade of CAR T-cell evolution. Nat Cancer. 2022;3:270–1. - PubMed
-
- Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T-cell survival in the tumor. Nat Biotechnol. 2018;36:346–51. - PubMed
-
- Zhao Z, Xiao X, Saw PE, Wu W, Huang H, Chen J, et al. Chimeric antigen receptor T cells in solid tumors: a war against the tumor microenvironment. Sci China Life Sci. 2020;63:180–205. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

