NF1 expression profiling in IDH-wildtype glioblastoma: genomic associations and survival outcomes
- PMID: 39472976
- PMCID: PMC11520828
- DOI: 10.1186/s40478-024-01875-z
NF1 expression profiling in IDH-wildtype glioblastoma: genomic associations and survival outcomes
Abstract
Background: NF1 inactivation is associated with sensitivity to MEK inhibitor targeted therapy in low-grade and some high-grade gliomas. NF1 loss may also be a harbinger of exploitable vulnerabilities in IDH-wildtype glioblastoma (GBM). Accurate and consistent detection of NF1 loss, however, is fraught given the large gene size, challenges with complete coverage and variant calling upon sequencing, and mechanisms of mRNA and protein regulation that result in early degradation in the absence of genomic alterations. Here, we seek to perform a composite analysis for NF1 loss accounting for genomic alterations and protein expression via immunohistochemistry. We also characterize the landscape of NF1 alterations in GBM.
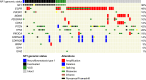
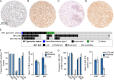
Methods: We assembled a single-institution, retrospective cohort of 542 IDH-wildtype GBM with somatic next generation sequencing to investigate the frequency and nature of detected NF1 alterations. We selected 69 GBMs from which to build a tissue microarray (TMA) of 44 NF1-wildtype and 25 NF1-mutant cases. We performed NF1 immunohistochemistry using two different NF1 antibodies (NFC, Sigma-Aldrich; and iNF-07E, iNFixion Bioscience) and correlated results with clinical, genomic, and other immunohistochemical features.
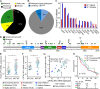
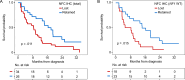
Results: In our retrospective cohort, we identified 88 IDH-wildtype GBM with NF1 alterations (16%). NF1 alterations were mutually exclusive with EGFR and MDM2 alterations (p-adj < 0.001, 0.05, respectively), but co-occurred with PIK3R1 alterations (Log2(OR) = - 1.6, p-adj = 0.03). Of the 63 scorable sporadic GBMs in the TMA, 14 harbored NF1 inactivating alterations and of those, 12 (86%) demonstrated minimal NF1 immunoreactivity by NFC antibody, compared to 8 (57%) by iNF-07E antibody. Among the 42 scorable NF1-wildtype GBM in the TMA, NF1 immunostaining was minimal in 18 (43%) by NFC antibody compared to 4 (10%) by iNF-07E antibody, potentially reflecting false positives or differential protein regulation. Minimal immunoreactivity by NFC antibody was associated with decreased median overall survival (8.5 vs. 16.4 months, p = 0.011). Cox proportional hazards model correcting for prognostic variables in this subset revealed HR 3.23 (95% CI 1.29-8.06, p = 0.01) associated with decreased NF1 expression by IHC.
Conclusion: NF1 immunostaining may serve as a sensitive surrogate marker of NF1 genomic inactivation and a valuable extension to next-generation sequencing for defining NF1 status. Minimal NF1 immunoreactivity is a poor prognostic marker, even in IDH-wildtype glioblastoma without apparent NF1 genomic alterations, but the underlying molecular mechanism requires further investigation.
Keywords: Biomarker; Glioblastoma; High-grade glioma; NF1; Neurofibromin.
© 2024. The Author(s).
Conflict of interest statement
CB is a consultant for Depuy-Synthe, Bionaut Labs, Haystack Oncology and Privo Technologies. CB is a co-founder of OrisDx and Belay Diagnostics. iNF-07E monoclonal antibody is owned and supplied by Infixion Bioscience, Inc. (San Diego). KCS has received honoraria from Novartis and institutional research funding from Springworks Therapeutics, Pfizer, Lantern Pharma, and FORE Biotherapeutics. She also sits on a data safety and monitoring board for Advarra.
Figures
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996 - PubMed
-
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352(10):997–1003 - PubMed
-
- Wen PY, Stein A, van den Bent M, De Greve J, Wick A, de Vos FYFL et al (2022) Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol 23(1):53–64 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

