Targeted hematopoietic stem cell depletion through SCF-blockade
- PMID: 39473008
- PMCID: PMC11523590
- DOI: 10.1186/s13287-024-03981-0
Targeted hematopoietic stem cell depletion through SCF-blockade
Abstract
Background: Hematopoietic stem cell transplantation (HSCT) is a curative treatment for many diverse blood and immune diseases. However, HSCT regimens currently commonly utilize genotoxic chemotherapy and/or total body irradiation (TBI) conditioning which causes significant morbidity and mortality through inducing broad tissue damage triggering infections, graft vs. host disease, infertility, and secondary cancers. We previously demonstrated that targeted monoclonal antibody (mAb)-based HSC depletion with anti(α)-CD117 mAbs could be an effective alternative conditioning approach for HSCT without toxicity in severe combined immunodeficiency (SCID) mouse models, which has prompted parallel clinical αCD117 mAbs to be developed and tested as conditioning agents in clinical trials starting with treatment of patients with SCID. Subsequent efforts have built upon this work to develop various combination approaches, though none are optimal and how any of these mAbs fully function is unknown.
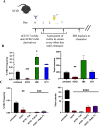
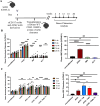
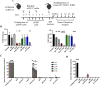
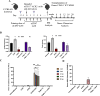
Methods: To improve efficacy of mAb-based conditioning as a stand-alone conditioning approach for all HSCT settings, it is critical to understand the mechanistic action of αCD117 mAbs on HSCs. Here, we compare the antagonistic properties of αCD117 mAb clones including ACK2, 2B8, and 3C11 as well as ACK2 fragments in vitro and in vivo in both SCID and wildtype (WT) mouse models. Further, to augment efficacy, combination regimens were also explored.
Results: We confirm that only ACK2 inhibits SCF binding fully and prevents HSC proliferation in vitro. Further, we verify that this corresponds to HSC depletion in vivo and donor engraftment post HSCT in SCID mice. We also show that SCF-blocking αCD117 mAb fragment derivatives retain similar HSC depletion capacity with enhanced engraftment post HSCT in SCID settings, but only full αCD117 mAb ACK2 in combination with αCD47 mAb enables enhanced donor HSC engraftment in WT settings, highlighting that the Fc region is not required for single-agent efficacy in SCID settings but is required in immunocompetent settings. This combination was the only non-genotoxic conditioning approach that enabled robust donor engraftment post HSCT in WT mice.
Conclusion: These findings shed new insights into the mechanism of αCD117 mAb-mediated HSC depletion. Further, they highlight multiple approaches for efficacy in SCID settings and optimal combinations for WT settings. This work is likely to aid in the development of clinical non-genotoxic HSCT conditioning approaches that could benefit millions of people world-wide.
© 2024. The Author(s).
Conflict of interest statement
A.C. discloses financial interests in the following entities working in the rare genetic disease space: Beam Therapeutics, Decibel Therapeutics, Editas Medicines, GV, Inograft Biotherapeutics, Prime Medicines, and Spotlight Therapeutics. In addition, she is an inventor on patents licensed to Decibel Therapeutics, Editas Medicines, Inograft Biotherapeutics, Jasper Therapeutics, and Magenta Therapeutics.
Figures


References
-
- Hawkins MM. Long-term survivors of childhood cancers: what knowledge have we gained? Nat Rev Clin Oncol. 2004;1:26–31. - PubMed
-
- Shimoni A, Shem-Tov N, Chetrit A, Volchek Y, Tallis E, Avigdor A, et al. Secondary malignancies after allogeneic stem-cell transplantation in the era of reduced-intensity conditioning; the incidence is not reduced. Leukemia. 2013;27:829–35. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

