A pilot study of AI-assisted reading of prostate MRI in Organized Prostate Cancer Testing
- PMID: 39473176
- PMCID: PMC11541807
- DOI: 10.2340/1651-226X.2024.40475
A pilot study of AI-assisted reading of prostate MRI in Organized Prostate Cancer Testing
Abstract
Objectives: To evaluate the feasibility of AI-assisted reading of prostate magnetic resonance imaging (MRI) in Organized Prostate cancer Testing (OPT).
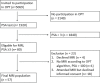
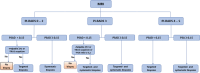
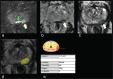
Methods: Retrospective cohort study including 57 men with elevated prostate-specific antigen (PSA) levels ≥3 µg/L that performed bi-parametric MRI in OPT. The results of a CE-marked deep learning (DL) algorithm for prostate MRI lesion detection were compared with assessments performed by on-site radiologists and reference radiologists. Per patient PI-RADS (Prostate Imaging-Reporting and Data System)/Likert scores were cross-tabulated and compared with biopsy outcomes, if performed. Positive MRI was defined as PI-RADS/Likert ≥4. Reader variability was assessed with weighted kappa scores.
Results: The number of positive MRIs was 13 (23%), 8 (14%), and 29 (51%) for the local radiologists, expert consensus, and DL, respectively. Kappa scores were moderate for local radiologists versus expert consensus 0.55 (95% confidence interval [CI]: 0.37-0.74), slight for local radiologists versus DL 0.12 (95% CI: -0.07 to 0.32), and slight for expert consensus versus DL 0.17 (95% CI: -0.01 to 0.35). Out of 10 cases with biopsy proven prostate cancer with Gleason ≥3+4 the DL scored 7 as Likert ≥4.
Interpretation: The Dl-algorithm showed low agreement with both local and expert radiologists. Training and validation of DL-algorithms in specific screening cohorts is essential before introduction in organized testing.
Figures
References
-
- Winkel DJ, Tong A, Lou B, Kamen A, Comaniciu D, Disselhorst JA, et al. . A novel deep learning based computer-aided diagnosis system improves the accuracy and efficiency of radiologists in reading biparametric magnetic resonance images of the prostate: results of a multireader, multicase study. Invest Radiol. 2021;56:605–13. 10.1097/RLI.0000000000000780 - DOI - PubMed
-
- Válek, V. Council recommendation of 9 December 2022 on strengthening prevention through early detection: a new EU approach on cancer screening. Off J Eur Union. C, 2022, 473..
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

