Safety Profile of Statins for Post-Marketing Adverse Cardiovascular Events: A Real-World Pharmacovigilance Analysis
- PMID: 39473255
- PMCID: PMC12376121
- DOI: 10.2174/0118715303324204240905111835
Safety Profile of Statins for Post-Marketing Adverse Cardiovascular Events: A Real-World Pharmacovigilance Analysis
Abstract
Aims and objectives: The purpose of this study was to comprehensively evaluate the association of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) with neurological adverse events using the US Food and Drug Administration Adverse Event Reporting System (FAERS) database, with the aim of guiding the rational use of statins.
Methods: The number and clinical characteristics of adverse events (AEs) to statins in the FAERS database between 2012 and March, 2023, were extracted. Neurological AEs were defined by the system organ classes (SOCs) of "Nervous System Disorders (10029205)" and the corresponding PT. Disproportionality was calculated using the reporting dominance ratio (ROR), proportional reporting ratio (PRR), and information component (IC025).
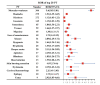
Results: Between January, 2012 and March, 2023, a total of 90,357 AEs were reported for the three statins (atorvastatin, resuvastatin, and simvastatin). The majority of reports on AEs came from the United States (n = 7284). A total of 8409 reports described neurological AEs following the use of the three statins, with atorvastatin accounting for more than half of the reports (n = 4430). The mean age of patients who developed neurological AEs was 55 years and older. The prevalence was similar in female patients (2230/4480) and male patients (1999/4480). Disproportionate analyses showed that at the SOC level, only the correlation between atorvastatin and neurological AEs suggested a positive signal (ROR: 9.77 (9.56-9.99); IC025: 3.28; PRR (χ2): 9.76 (16.07)) and in total, there were 32 PTs with a positive signal. The median time for neurological AEs was 71 days (IQR: 14-559 days), and the most common AEs were other serious effects (important medical event) (OT) (n = 2283) and hospitalization (HO) (n = 715).
Conclusion: This study suggests that atorvastatin may be associated with an increased risk of neurological AEs. This study provides realistic evidence of the potential risk of statin-related adverse events.
Keywords: FDA Adverse Event Reporting System; Statins; adverse events; atorvastatin; neurological; real-world study..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures


References
-
- Ray K.K., Nicholls S.J., Li N., Louie M.J., Brennan D., Lincoff A.M., Nissen S.E. Efficacy and safety of bempedoic acid among patients with and without diabetes: Prespecified analysis of the CLEAR Outcomes randomised trial. Lancet Diabetes Endocrinol. 2024;12(1):19–28. doi: 10.1016/S2213-8587(23)00316-9. - DOI - PubMed
-
- Walker A.J., Zhu J., Thoma F., Marroquin O., Makani A., Gulati M., Gianos E., Virani S.S., Rodriguez F., Reis S.E., Ballantyne C., Mulukutla S., Saeed A. Statin utilization and cardiovascular outcomes in a real-world primary prevention cohort of older adults. Am. J. Prevent. Cardiol. 2024;18:100664. doi: 10.1016/j.ajpc.2024.100664. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

