Effects of mesenchymal stromal cells and human recombinant Nerve Growth Factor delivered by bioengineered human corneal lenticule on an innovative model of diabetic retinopathy
- PMID: 39473506
- PMCID: PMC11518713
- DOI: 10.3389/fendo.2024.1462043
Effects of mesenchymal stromal cells and human recombinant Nerve Growth Factor delivered by bioengineered human corneal lenticule on an innovative model of diabetic retinopathy
Abstract
Introduction: Diabetic retinopathy (DR) is a microvascular complication of diabetes in which neurodegeneration has been recently identified as a driving force. In the last years, mesenchymal stromal cells (MSCs) and neurotrophins like Nerve Growth Factor (NGF), have garnered significant attention as innovative therapeutic approaches targeting DR-associated neurodegeneration. However, delivering neurotrophic factors directly in the eye remains a challenge. Hence, this study evaluated the effects of MSCs from human amniotic fluids (hAFSCs) and recombinant human NGF (rhNGF) delivered by human corneal lenticule (hCL) on a high glucose (HG) induced ex vivo model simulating the molecular mechanisms driving DR.
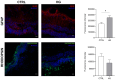
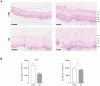
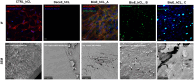
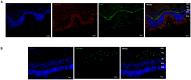
Methods: Porcine neuroretinal explants exposed to HG (25 mM for four days) were used to mimic DR ex vivo. hCLs collected from donors undergoing refractive surgery were decellularized using 0.1% sodium dodecyl sulfate and then bioengineered with hAFSCs, microparticles loaded with rhNGF (rhNGF-PLGA-MPs), or both simultaneously. Immunofluorescence (IF) and scanning electron microscopy (SEM) analyses were performed to confirm the hCLs bioengineering process. To assess the effects of hAFSCs and rhNGF, bioengineered hCLs were co-cultured with HG-treated neuroretinal explants and following four days RT-PCR and cytokine array experiments for inflammatory, oxidative, apoptotic, angiogenic and retinal cells markers were performed.
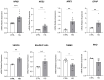
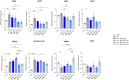
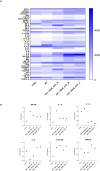
Results: Data revealed that HG-treated neuroretinal explants exhibit a characteristic DR-phenotype, including increased level of NF-kB, NOS2, NRF2 GFAP, VEGFA, Bax/Bcl2 ratio and decreased expression of TUBB3 and Rho. Then, the feasibility to bioengineer decellularized hCLs with hAFSCs and rhNGF was demonstrated. Interestingly, co-culturing hAFSCs- and rhNGF- bioengineered hCLs with HG-treated neuroretinal explants for four days significantly reduced the expression of inflammatory, oxidative, apoptotic, angiogenic and increased retinal markers.
Conclusion: Overall, we found for the first time that hAFSCs and rhNGF were able to modulate the molecular mechanisms involved in DR and that bioengineered hCLs represents a promising ocular drug delivery system of hAFSCs and rhNGF for eye diseases treatment. In addition, results demonstrated that porcine neuroretinal explants treated with HG is a useful model to reproduce ex vivo the DR pathophysiology.
Keywords: corneal lenticule; diabetic retinopathy; mesenchymal stromal cells; ocular delivery; rhNGF.
Copyright © 2024 Pelusi, Hurst, Detta, Pipino, Lamolinara, Conte, Mastropasqua, Allegretti, Di Pietrantonio, Romeo, El Zarif, Nubile, Guerricchio, Bollini, Pandolfi, Schnichels and Mandatori.
Conflict of interest statement
The authors ND, GC, MA, and TR are employed by Dompé Farmaceutici SpA. ND, MA, MN, AP, and DM are authors of European Patent n° 20179055.7-1109, 09/06/2020, “New Drug Delivery System for Ophtalmic Use”. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

