Copper-Catalyzed Enantioconvergent Radical C(sp3)-N Cross-Coupling to Access Chiral α-Amino-β-lactams
- PMID: 39473577
- PMCID: PMC11503772
- DOI: 10.1021/prechem.3c00084
Copper-Catalyzed Enantioconvergent Radical C(sp3)-N Cross-Coupling to Access Chiral α-Amino-β-lactams
Abstract
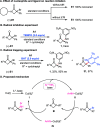
A copper-catalyzed enantioconvergent radical C(sp3)-N cross-coupling of racemic tertiary α-bromo-β-lactams with aromatic amines is developed under mild thermal reaction conditions. The use of a sterically demanded oxazoline-derived sulfonamide N,N,N-ligand is crucial for the reaction initiation and effective enantio-discrimination of the azetidinone-derived cyclic alkyl radicals. The strategy provides an attractive approach to access chiral α-amino-β-lactams, an important structural motif in many biologically active molecules. Preliminary mechanistic studies support the formation of azetidinone-derived alkyl radicals from the L*Cu(I)-amido complex and α-bromo-β-lactams.
© 2023 The Authors. Co-published by University of Science and Technology of China and American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Brandi A.; Cicchi S.; Cordero F. M. Novel Syntheses of Azetidines and Azetidinones. Chem. Rev. 2008, 108, 3988–4035. 10.1021/cr800325e. - DOI - PubMed
- Pitts C. R.; Lectka T. Chemical Synthesis of β-Lactams: Asymmetric Catalysis and Other Recent Advances. Chem. Rev. 2014, 114, 7930–7953. 10.1021/cr4005549. - DOI - PubMed
- Decuyper L.; Jukič M.; Sosič I.; Žula A.; D’hooghe M.; Gobec S. Antibacterial and β-Lactamase Inhibitory Activity of Monocyclic β-Lactams. Med. Res. Rev. 2018, 38, 426–503. 10.1002/med.21443. - DOI - PubMed
- da Silveira Pinto L. S.; Alves Vasconcelos T. R.; Gomes C. R. B.; de Souza M. V. N. A Brief Review on the Development of Novel Potentially Active Azetidin-2-ones Against Cancer. Curr. Org. Chem. 2020, 24, 473–486. 10.2174/1385272824666200303115444. - DOI
-
- Ojima I.; Delaloge F. Asymmetric Synthesis of Building-Blocks for Peptides and Peptidomimetics by Means of the β-Lactam Synthon Method. Chem. Soc. Rev. 1997, 26, 377–386. 10.1039/CS9972600377. - DOI
- Alcaide B.; Almendros P.; Aragoncillo C. β-Lactams: Versatile Building Blocks for the Stereoselective Synthesis of Non-β-Lactam Products. Chem. Rev. 2007, 107, 4437–4492. 10.1021/cr0307300. - DOI - PubMed
- Palomo C.; Oiarbide M. β-Lactam Ring Opening: A Useful Entry to Amino Acids and Relevant Nitrogen-Containing Compounds. Top. Heterocycl. Chem. 2010, 22, 211–259. 10.1007/7081_2009_11. - DOI
-
-
For selected examples, see:
- Hodous B. L.; Fu G. C. Enantioselective Staudinger Synthesis of β-Lactams Catalyzed by a Planar-Chiral Nucleophile. J. Am. Chem. Soc. 2002, 124, 1578–1579. 10.1021/ja012427r. - DOI - PubMed
- Lee E. C.; Hodous B. L.; Bergin E.; Shih C.; Fu G. C. Catalytic Asymmetric Staudinger Reactions to Form β-Lactams: An Unanticipated Dependence of Diastereoselectivity on the Choice of the Nitrogen Substituent. J. Am. Chem. Soc. 2005, 127, 11586–11587. 10.1021/ja052058p. - DOI - PubMed
- Tong H.-R.; Zheng W.; Lv X.; He G.; Liu P.; Chen G. Asymmetric Synthesis of β-Lactam via Palladium-Catalyzed Enantioselective Intramolecular C(sp3)-H Amidation. ACS Catal. 2020, 10, 114–120. 10.1021/acscatal.9b04768. - DOI
- Qi J.; Wei F.; Huang S.; Tung C.-H.; Xu Z. Copper(I)-Catalyzed Asymmetric Interrupted Kinugasa Reaction: Synthesis of α-Thiofunctional Chiral β-Lactams. Angew. Chem., Int. Ed. 2021, 60, 4561–4565. 10.1002/anie.202013450. - DOI - PubMed
- Qi J.; Wei F.; Tung C.-H.; Xu Z. Modular Synthesis of α-Quaternary Chiral β-Lactams by a Synergistic Copper/Palladium-Catalyzed Multicomponent Reaction. Angew. Chem., Int. Ed. 2021, 60, 13814–13818. 10.1002/anie.202100601. - DOI - PubMed
- Zhong F.; Yue W.-J.; Yin X.-H.; Zhang H.-M.; Yin L. Copper(I)-Catalyzed Asymmetric Synthesis of α-Allenylamines and β-Lactams through Regioselective Mannich-Type Reactions. ACS Catal. 2022, 12, 9181–9189. 10.1021/acscatal.2c01399. - DOI
- Qi J.; Song T.; Yang Z.; Sun S.; Tung C.-H.; Xu Z. Simultaneous Dual Cu/Ir Catalysis: Stereodivergent Synthesis of Chiral β-Lactams with Adjacent Tertiary/Quaternary/Tertiary Stereocenters. ACS Catal. 2023, 13, 2555–2564. 10.1021/acscatal.2c04926. - DOI
-
-
-
For a recent review, see:
- Deketelaere S.; Van Nguyen T.; Stevens C. V.; D’hooghe M. Synthetic Approaches toward Monocyclic 3-Amino-β-Lactams. ChemistryOpen 2017, 6, 301–319. 10.1002/open.201700051. - DOI - PMC - PubMed
-
For selected examples, see:
- Decuyper L.; Magdalenić K.; Verstraete M.; Jukič M.; Sosič I.; Sauvage E.; Amoroso A. M.; Verlaine O.; Joris B.; Gobec S.; D’hooghe M. α-Unsaturated 3-Amino-1-carboxymethyl-β-lactams as Bacterial PBP Inhibitors: Synthesis and Biochemical Assessment. Chem.—Eur. J. 2019, 25, 16128–16140. 10.1002/chem.201904139. - DOI - PubMed
- Grabrijan K.; Strašek N.; Gobec S. Synthesis of 3-Amino-4-substituted Monocyclic β-Lactams-Important Structural Motifs in Medicinal Chemistry. Int. J. Mol. Sci. 2022, 23, 360.10.3390/ijms23010360. - DOI - PMC - PubMed
-
-
- Fleming A. On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to Their Use in the Isolation of B. influenzae. Br. J. Exp. Pathol. 1929, 10, 226–236.
LinkOut - more resources
Full Text Sources