Tetrabromobisphenol A-bis(2,3-dibromopropyl ether) impairs Postnatal Testis Development in Mice: The Microtubule Cytoskeleton as a Sensitive Target
- PMID: 39473615
- PMCID: PMC11504584
- DOI: 10.1021/envhealth.3c00044
Tetrabromobisphenol A-bis(2,3-dibromopropyl ether) impairs Postnatal Testis Development in Mice: The Microtubule Cytoskeleton as a Sensitive Target
Abstract
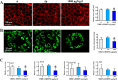
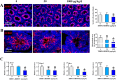
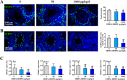
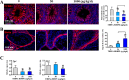
Tetrabromobisphenol A-bis(2,3-dibromopropyl ether) (TBBPA-BDBPE), a widely used flame retardant, has been frequently detected in various environmental compartments, but its health hazard remains largely unknown. Here, we investigated the adverse effects of TBBPA-BDBPE (50 and 1000 μg/kg/day) on postnatal testis development in CD-1 mice and the underlying mechanism. Following the first week of maternal exposure, neonatal mice in the high-dose group exhibited reduced seminiferous tubule area, fewer Sertoli cells and germ cells, and damaged microtubules in Sertoli cells; even microtubule damage was also observed in the low-dose group. When exposure extended to adulthood, male offspring in the high-dose group presented more remarkable alterations in reproductive parameters, including reduced sperm count; in the low-dose group, microtubule damage was also observable, along with blood-testis barrier impairment. Further molecular docking analysis and tubulin polymerization assay indicated that TBBPA-BDBPE could interact with tubulin and disrupt its polymerization. Moreover, we observed attenuated microtubules in mouse Sertoli cells in vitro (TM4) following TBBPA-BDBPE treatment, suggesting that TBBPA-BDBPE impaired testis development possibly by interfering with tubulin dynamics. This study not only highlights the male reproductive hazard of TBBPA-BDBPE but also greatly improved the understanding of the molecular mechanism for male reproductive toxicity of chemicals.
© 2023 The Authors. Co-published by Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, and American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Extended exposure to tetrabromobisphenol A-bis(2,3-dibromopropyl ether) leads to subfertility in male mice at the late reproductive age.Arch Toxicol. 2023 Nov;97(11):2983-2995. doi: 10.1007/s00204-023-03589-y. Epub 2023 Aug 22. Arch Toxicol. 2023. PMID: 37606655
-
Tetrabromobisphenol-A-Bis(dibromopropyl ether) Flame Retardant in Eggs, Regurgitates, and Feces of Herring Gulls from Multiple North American Great Lakes Locations.Environ Sci Technol. 2019 Aug 20;53(16):9564-9571. doi: 10.1021/acs.est.9b02472. Epub 2019 Jul 31. Environ Sci Technol. 2019. PMID: 31364365
-
Toxicity of Tetrabromobisphenol A and Its Derivative in the Mouse Liver Following Oral Exposure at Environmentally Relevant Levels.Environ Sci Technol. 2021 Jun 15;55(12):8191-8202. doi: 10.1021/acs.est.1c01726. Epub 2021 Jun 4. Environ Sci Technol. 2021. PMID: 34086441
-
Tetrabromobisphenol A (TBBPA): A controversial environmental pollutant.J Environ Sci (China). 2020 Nov;97:54-66. doi: 10.1016/j.jes.2020.04.039. Epub 2020 Jun 7. J Environ Sci (China). 2020. PMID: 32933740 Review.
-
[Tetrabromobisphenol A - Toxicity, environmental and occupational exposures].Med Pr. 2017 Feb 28;68(1):121-134. doi: 10.13075/mp.5893.00491. Epub 2017 Feb 27. Med Pr. 2017. PMID: 28245009 Review. Polish.
Cited by
-
Bioaccumulation and Maternal Transfer of Brominated Flame Retardants in Poultry and the Health Risks from Dietary Exposure.Environ Health (Wash). 2025 Mar 17;3(7):723-732. doi: 10.1021/envhealth.4c00260. eCollection 2025 Jul 18. Environ Health (Wash). 2025. PMID: 40704072 Free PMC article.
References
-
- An alternatives assessment for the flame retardant decabromodiphenyl ether (DecaBDE); EPA: Washington, DC, 2014.
-
- Flame retardant alternatives for hexabromocyclododecane (HBCD); EPA: Washington, DC, 2014.
LinkOut - more resources
Full Text Sources
Miscellaneous
