Optimizing gefitinib nanoliposomes by Box-Behnken design and coating with chitosan: A sequential approach for enhanced drug delivery
- PMID: 39473624
- PMCID: PMC11517515
- DOI: 10.5599/admet.2366
Optimizing gefitinib nanoliposomes by Box-Behnken design and coating with chitosan: A sequential approach for enhanced drug delivery
Abstract
Background and purpose: This study aimed to improve the stability and prolonged gefitinib release from the nanoliposomes.
Experimental approach: Nanoliposomes were prepared by reverse-phase evaporation and optimized using Box-Behnken design to investigate the influence of sonication time (X 1), tween 80 / soya phosphatidylcholine ratio (X 2), and cholesterol/soya phosphatidylcholine ratio (X 3) on nanoliposomes.
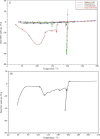
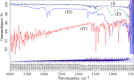
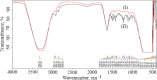
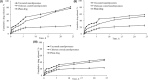
Key results: Optimized nanoliposomes were quasi-spherical shaped, with a mean dimension of 93.2 nm and an encapsulation efficiency of 87.56±0.17 %. Surface decoration of the optimized batch was done using different concentrations of chitosan. The optimal chitosan concentration required to adorn the nanoliposome surface was 0.01 %. In comparison to unadorned nanoliposomes (82.16±0.65 %), adorned nanoliposomes (78.04±0.35 %) released the drug consistently over 24 h via Fickian diffusion. The IC50 values for surface-adorned nanoliposomes in A549 and H1299 cells were 6.53±0.75 and 4.73±0.46 μM, respectively. Cytotoxicity of the surface-decorated nanoliposomes may be due to their higher zeta potential and prolonged drug release. At the end of the sixth month, the samples stored at 4 °C were more stable than those stored at 25 °C and 45 °C. The stability of plain nanoliposomes has increased after chitosan coating. Thus, by using different concentrations of chitosan solution as coating material, we can develop a suitable sustained drug-release surface-adorned nanoliposomal formulation.
Conclusion: The developed nanoliposomes may offer a new path for melanoma clinics.
Keywords: Liposomes; pulmonary cancer; response surface methodology.
Copyright © 2024 by the authors.
Conflict of interest statement
Conflict of interest: The authors declared that there are no conflicts of interest.
Figures




Similar articles
-
Novel Chitosan-Coated Liposomes Coloaded with Exemestane and Genistein for an Effective Breast Cancer Therapy.ACS Omega. 2024 Feb 16;9(8):9735-9752. doi: 10.1021/acsomega.3c09948. eCollection 2024 Feb 27. ACS Omega. 2024. PMID: 38434864 Free PMC article.
-
Formulation of chitosan coated nanoliposomes for the oral delivery of colistin sulfate: in vitro characterization, 99mTc-radiolabeling and in vivo biodistribution studies.Drug Dev Ind Pharm. 2021 Apr;47(4):626-635. doi: 10.1080/03639045.2021.1908334. Epub 2021 Apr 9. Drug Dev Ind Pharm. 2021. PMID: 33834934
-
Modifying the Stability and Surface Characteristic of Anthocyanin Compounds Incorporated in the Nanoliposome by Chitosan Biopolymer.Pharmaceutics. 2022 Aug 3;14(8):1622. doi: 10.3390/pharmaceutics14081622. Pharmaceutics. 2022. PMID: 36015248 Free PMC article.
-
Formulation and characterization of alprazolam-loaded nanoliposomes: screening of process variables and optimizing characteristics using RSM.Drug Dev Ind Pharm. 2018 Feb;44(2):296-305. doi: 10.1080/03639045.2017.1391834. Epub 2017 Nov 2. Drug Dev Ind Pharm. 2018. PMID: 29022858
-
Probing nanoliposomes using single particle analytical techniques: effect of excipients, solvents, phase transition and zeta potential.Heliyon. 2018 Dec 26;4(12):e01088. doi: 10.1016/j.heliyon.2018.e01088. eCollection 2018 Dec. Heliyon. 2018. PMID: 30603716 Free PMC article. Review.
Cited by
-
Fabrication and optimization of freeze-dried isoniazid-loaded poly-ε-caprolactone nanoparticles.ADMET DMPK. 2025 Jul 8;13(4):2774. doi: 10.5599/admet.2774. eCollection 2025. ADMET DMPK. 2025. PMID: 40786068 Free PMC article.
-
Curcumin-loaded nanoemulsion for acute lung injury treatment via nebulization: Formulation, optimization and in vivo studies.ADMET DMPK. 2025 Mar 20;13(2):2661. doi: 10.5599/admet.2661. eCollection 2025. ADMET DMPK. 2025. PMID: 40314003 Free PMC article.
References
-
- Cryer A.M., Thorley A.J.. Nanotechnology in the diagnosis and treatment of lung cancer. Pharmacology & Therapeutics 198 (2019) 189-205. https://doi.org/10.1016/j.pharmthera.2019.02.010 10.1016/j.pharmthera.2019.02.010. - DOI - PubMed
-
- Rivera M.P., Mehta A.C., Wahidi M.M.. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer. Chest 143 (2013) e142S-e165S. https://doi.org/10.1378/chest.12-2353 10.1378/chest.12-2353. - DOI - PubMed
-
- Hochhegger B., Alves G.R., Irion K.L., Fritscher C.C., Fritscher L.G., Concatto N.H., Marchiori E.. PET/CT imaging in lung cancer: Indications and findings. Jornal Brasileiro de Pneumologia 41 (2015) 264-274. https://doi.org/10.1590/S1806-37132015000004479 10.1590/S1806-37132015000004479. - DOI - PMC - PubMed
-
- Carrasco-Esteban E., Dominguez-Rullan J.A., Barrionuevo-Castillo P., Pelari-Mici L., Leaman O., Sastre-Gallego S., López-Campos F.. Current role of nanoparticles in the treatment of lung cancer. Journal of Clinical and Translational Research 7 (2021) 140-155. https://doi.org/10.18053/jctres.07.202102.005 10.18053/jctres.07.202102.005. - DOI - PMC - PubMed
-
- Miller Y.E.. Pathogenesis of lung cancer. American Journal of Respiratory Cell and Molecular Biology 33 (2005) 216-223. https://doi.org/10.1165/rcmb.2005-0158OE 10.1165/rcmb.2005-0158OE. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
