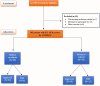
Role of Biomarkers Diagnostic Tools in Patients with COVID-19: Stratification Made Easy
- PMID: 39473634
- PMCID: PMC11520913
- DOI: 10.2147/IJGM.S488968
Role of Biomarkers Diagnostic Tools in Patients with COVID-19: Stratification Made Easy
Abstract
Background and aims: In coronavirus disease 2019 (COVID-19) patients, several serum biomarkers have been identified. Upon intensive care unit (ICU) admission, these laboratory markers become more crucial to distinguish between patients with severe cases of COVID-19. It might assist doctors in predicting the course of illnesses and treating patients appropriately. This work was to investigate the role of biomarkers in patients with COVID-19 classification admitted to the hospital and identified by reverse transcription polymerase chain reaction (RT-PCR).
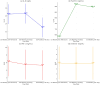
Methods: Peripheral blood sample was taken from COVID-19 cases isolated on admission to determine C-reactive protein (CRP), D-dimer, Fibrinogen, neutrophil-lymphocyte ratio (NLR), leukocytes CRP ratio (LeCR), lymphocyte-CRP ratio (LCR), interleukin-6 (IL6), leukocytes interleukin 6 ratio (LeIL6), systemic inflammatory index (SII), platelet-to-lymphocyte ratio (PLR), and tissue plasminogen activator inhibitor one (tPAI-1). Follow-up for IL6, Ferritin, D-dimer, and tPAI-1 were determined on the 3rd and 7th days.
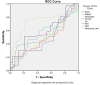
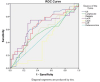
Results: Comparisons of severity revealed that hypertension, chronic obstructive pulmonary disease (COPD), and Ischemia were major risk factors in COVID-19 patients. There was a statistically significant difference between the test groups for fibrinogen (p < 0.000), IL6 (p < 0.009), LeCR (p < 0.006), and LCR (p < 0.011).
Conclusion: Based on laboratory test findings at the time of ICU admission, we can distinguish severe cases of COVID-19.
Keywords: COVID-19; RT- PCR; intensive care unit; risk factors; serum biomarkers.
© 2024 Salman et al.
Conflict of interest statement
The authors declare no competing interests in this work.
Figures
References
-
- Abensur Vuillaume L, Lefebvre F, Benhamed A, et al. CREMS network (clinical research in emergency medicine and sepsis). Lymphocyte-to-C-reactive protein (LCR) ratio is not accurate to predict severity and mortality in patients with COVID-19 admitted to the ED. Int J Mol Sci. 2023;24(6):5996. doi: 10.3390/ijms24065996 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

