Pathogen-Directed Antimicrobial Stewardship Audit Rounds: Evaluation of an Objectively Planned, Clinical Microbiology-Driven Antimicrobial Stewardship Approach to Influence Antimicrobial Prescriptions of Prescribers
- PMID: 39473648
- PMCID: PMC11521104
- DOI: 10.7759/cureus.70441
Pathogen-Directed Antimicrobial Stewardship Audit Rounds: Evaluation of an Objectively Planned, Clinical Microbiology-Driven Antimicrobial Stewardship Approach to Influence Antimicrobial Prescriptions of Prescribers
Abstract
Introduction: Correct and prompt antimicrobial prescription modifications by the treating team based on the culture and antimicrobial susceptibility test (AST) reports are crucial. Pathogen-directed antimicrobial stewardship (PD-AMS) audit rounds are a clinical microbiology-driven approach to combine prompt appropriate patient management goals with goals of preventing irrational and improper use of antimicrobials.
Methods: The study was a prospective before-after interventional study to evaluate the effectiveness of PD-AMS audit rounds in modifying antimicrobial usage by prescribers on an individual patient basis. It is divided into a two-month pre-intervention phase (no active advice is given with passive monitoring) and an intervention phase for four months, with antimicrobial therapy-related advice during PD-AMS rounds taken as "Intervention." Antimicrobial advice was objectively classified into various categories. The PD-AMS audit round was conducted for all inpatients with a culture-proven bloodstream infection with a pathogen, and objectively classified antimicrobial advice was given through bedside rounds, telephonically, and/or written advice forms. Compliance with the advice was monitored on the next day (or at the end of 24 hours) of the PD-AMS round.
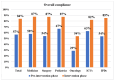
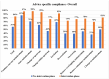
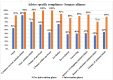
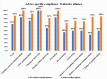
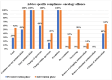
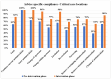
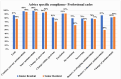
Results: The compliance with culture AST report regarding antimicrobial prescription modifications improved significantly in the intervention phase compared to the pre-intervention phase as follows: overall (83.8% (612/730) vs. 56.7% (242/427)); medicine alliance (87.4% (263/301) vs. 57.8% (115/199)); surgery alliance (87.2% (170/195) vs. 54.0% (87/161)); pediatric alliance (87.3% (138/158) vs. 66.7% (26/39)); oncology alliance (53.9% (41/76) vs. 28.6% (8/28)); critical care locations (82.0% (260/317) vs. 62.7% (89/142)); and in-patient departments (85.2% (352/413) vs. 53.7% (153/285)).
Conclusion: The clinical microbiology team should mandatorily conduct an objectively planned PD-AMS audit round to improve patient care and management and antimicrobial prescriptions through a mutual discussion with prescribers and to help address their doubts.
Keywords: antimicrobial advice; antimicrobial prescription modifications; antimicrobial stewardship; ast-guided antimicrobial therapy; bedside rounds; clinical microbiology; compliance of prescribers; culture-guided therapy; pathogen-directed therapy.
Copyright © 2024, Priyadarshi et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Jawaharlal Institute of Postgraduate Medical Education & Research issued approval JIP/IEC/2021/044. The study did not include the direct involvement of any human participant and hence had been provided a waiver of informed consent. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures



References
-
- Rapid identification and antimicrobial susceptibility testing reduce antibiotic use and accelerate pathogen-directed antibiotic use. Kerremans JJ, Verboom P, Stijnen T, Hakkaart-van Roijen L, Goessens W, Verbrugh HA, Vos MC. J Antimicrob Chemother. 2008;61:428–435. - PubMed
-
- Impact of a prospective-audit-with-feedback antimicrobial stewardship program at a children’s hospital. Newland JG, Stach LM, De Lurgio SA, et al. J Pediatric Infect Dis Soc. 2012;1:179–186. - PubMed
-
- Decreased antimicrobial resistance and defined daily doses after implementation of a clinical culture-guided antimicrobial stewardship program in a local hospital. Wu CT, Chen CL, Lee HY, et al. J Microbiol Immunol Infect. 2017;50:846–856. - PubMed
-
- The impact of a multidisciplinary antimicrobial stewardship team on the timeliness of antimicrobial therapy in patients with positive blood cultures: a randomized controlled trial. Cairns KA, Doyle JS, Trevillyan JM, et al. J Antimicrob Chemother. 2016;71:3276–3283. - PubMed
LinkOut - more resources
Full Text Sources
