Gilteritinib-Induced Hypopituitarism: A Case Report
- PMID: 39473682
- PMCID: PMC11518918
- DOI: 10.7759/cureus.70401
Gilteritinib-Induced Hypopituitarism: A Case Report
Abstract
Gilteritinib is a tyrosine kinase inhibitor (TKI) that treats acute myeloid leukemia (AML) by inhibiting FMS-like tyrosine kinase 3 (FLT3). This is a report on hypopituitarism induced by gilteritinib and its resolution following withdrawal. A 54-year-old woman was treated with gilteritinib for AML. She subsequently developed general fatigue. Blood tests showed low levels of anterior pituitary hormone. After 10 months of gilteritinib withdrawal, the levels of anterior pituitary hormones returned to normal values. When nonspecific symptoms such as fatigue in patients treated with gilteritinib are coupled with electrolyte abnormalities, a close checkup for hypopituitarism is recommended.
Keywords: acute myeloid leukemia; drug withdrawal; gilteritinib; hypopituitarism; pituitary hormone.
Copyright © 2024, Hori et al.
Conflict of interest statement
Human subjects: Consent was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
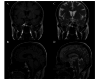
Figures

References
-
- Pharmacological and clinical profile of gilteritinib (Xospata® tablets 40 mg), a therapeutic agent for relapsed or refractory FLT3-mutated acute myeloid leukemia. Mori M, Hidaka K. Folia Pharmacol Jpn. 2021;156:37–46. - PubMed
-
- Zospata Tablets 40 mg Drug Interview HomeRevised. [ Jun; 2024 ]. 2023. https://amn.astellas.jp/common/pdfviewer.html/content/dam/jp/amn/jp/ja/d... https://amn.astellas.jp/common/pdfviewer.html/content/dam/jp/amn/jp/ja/d...
-
- Pharmacological and clinical profile of gilteritinib (Xospata(®) tablets 40 mg), a therapeutic agent for relapsed or refractory FLT3-mutated acute myeloid leukemia (Article in Japanese) Mori M, Hidaka K. Nihon Yakurigaku Zasshi. 2021;156:37–46. - PubMed
-
- Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. Perl AE, Martinelli G, Cortes JE, et al. N Engl J Med. 2022;386:1868. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
