Recognition of Single Fluorescence Events by Temporal Pixel Intensity Fluctuation
- PMID: 39473693
- PMCID: PMC11504586
- DOI: 10.1021/cbmi.3c00043
Recognition of Single Fluorescence Events by Temporal Pixel Intensity Fluctuation
Abstract
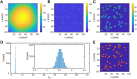
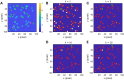
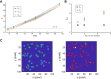
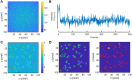
Single-molecule localization microscopy circumvents the diffraction limit of traditional fluorescence microscopy by detecting the photoemission signals of individual fluorescent molecules. The accurate recognitions of fluorescence molecules/events are critical to single-molecule/super-resolution imaging experiments, which determine the precision of molecular localizations and the quality of the image reconstruction. Herein, we presented a single-molecule detection method which relied on the temporal pixel intensity fluctuation. The method was capable of quickly determining the approximate localizations of fluorescence events with high sensitivity. We evaluated the performance of the method under a series of signal-to-noise ratios (SNR) and discussed the criterion of setting the temporal fluctuation threshold to achieve the optimized spots recognition results.
© 2023 The Authors. Co-published by Nanjing University and American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Parameter-free rendering of single-molecule localization microscopy data for parameter-free resolution estimation.Commun Biol. 2021 May 11;4(1):550. doi: 10.1038/s42003-021-02086-1. Commun Biol. 2021. PMID: 33976358 Free PMC article.
-
Fisher information and the Cramér-Rao lower bound in single-pixel localization microscopy with spatiotemporally modulated illumination.J Opt Soc Am A Opt Image Sci Vis. 2023 Jan 1;40(1):185-203. doi: 10.1364/JOSAA.480015. J Opt Soc Am A Opt Image Sci Vis. 2023. PMID: 36607089
-
Superior performance with sCMOS over EMCCD in super-resolution optical fluctuation imaging.J Biomed Opt. 2016 Jun 1;21(6):66007. doi: 10.1117/1.JBO.21.6.066007. J Biomed Opt. 2016. PMID: 27281064
-
[Comparison and progress review of various super-resolution fluorescence imaging techniques].Se Pu. 2021 Oct;39(10):1055-1064. doi: 10.3724/SP.J.1123.2021.06015. Se Pu. 2021. PMID: 34505427 Free PMC article. Review. Chinese.
-
Recent advances in super-resolution fluorescence imaging and its applications in biology.J Genet Genomics. 2013 Dec 20;40(12):583-95. doi: 10.1016/j.jgg.2013.11.003. Epub 2013 Nov 23. J Genet Genomics. 2013. PMID: 24377865 Review.
References
-
- Wang Y.; Kuang C.; Cai H.; Li S.; Liu W.; Hao X.; Ge J.; Liu X. Sub-diffraction imaging with confocal fluorescence microscopy by stochastic photobleaching. Opt. Commun. 2014, 312, 62–67. 10.1016/j.optcom.2013.09.022. - DOI
LinkOut - more resources
Full Text Sources
