eHealth and mHealth in Antimicrobial Stewardship Programs
- PMID: 39473804
- PMCID: PMC11521536
- DOI: 10.1159/000541120
eHealth and mHealth in Antimicrobial Stewardship Programs
Abstract
Background: The global need for rapid diagnostic methods for pathogen identification and antimicrobial susceptibility testing (AST) is underscored by the increasing bacterial resistance and limited therapeutic options, especially critical in sepsis management.
Summary: This review examines the aspects of the eHealth and mHealth in Antimicrobial Stewardship Programs (ASPs) to improve the treatment of infections and rational use of antimicrobials.
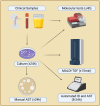
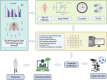
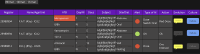
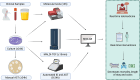
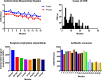
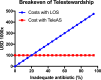
Key messages: The evolution from traditional phenotype-based methods to rapid molecular and mass spectrometry techniques has significantly decreased result turnaround times, improving patient outcomes. Despite advancements, the complex decision-making in antimicrobial therapy often exceeds the capacity of many clinicians, highlighting the importance of ASPs. These programs, integrating mHealth and eHealth, leverage technology to enhance healthcare services and patient outcomes, particularly in remote or resource-limited settings. However, the application of such technologies in antimicrobial management remains underexplored in hospitals. The development of platforms combining antimicrobial prescription data with pharmacotherapeutic algorithms and laboratory integration can significantly reduce costs and improve hospitalization times and mortality rates.
Keywords: Antimicrobial resistance; Antimicrobial stewardship; Pharmacotherapeutic algorithms; Rapid diagnostic methods; mHealth and eHealth.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Figures
Similar articles
-
eHealth and mHealth in Antimicrobial Stewardship to Reduce Mortality in Empirical Antimicrobial Therapy and a Systematic Review with a Meta-Analysis of Adequate Therapy.Infect Dis Rep. 2024 Aug 1;16(4):707-723. doi: 10.3390/idr16040054. Infect Dis Rep. 2024. PMID: 39195005 Free PMC article. Review.
-
Opportunities to Enhance Diagnostic Testing and Antimicrobial Stewardship: A Qualitative Multinational Survey of Healthcare Professionals.Infect Dis Ther. 2024 Jul;13(7):1621-1637. doi: 10.1007/s40121-024-00996-1. Epub 2024 Jun 3. Infect Dis Ther. 2024. PMID: 38829440 Free PMC article.
-
Going digital: a narrative overview of the clinical and organisational impacts of eHealth technologies in hospital practice.Aust Health Rev. 2017 Dec;41(6):646-664. doi: 10.1071/AH16233. Aust Health Rev. 2017. PMID: 28063462 Review.
-
Pediatric Antimicrobial Stewardship: State of the Art.Curr Infect Dis Rep. 2018 Aug 1;20(10):39. doi: 10.1007/s11908-018-0644-7. Curr Infect Dis Rep. 2018. PMID: 30069834 Review.
-
Rapid antimicrobial susceptibility tests for sepsis; the road ahead.J Med Microbiol. 2019 Jul;68(7):973-977. doi: 10.1099/jmm.0.000997. Epub 2019 May 30. J Med Microbiol. 2019. PMID: 31145055
References
-
- Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. . Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176–87. - PubMed
-
- Wenzler E, Maximos M, Asempa TE, Biehle L, Schuetz AN, Hirsch EB. Antimicrobial susceptibility testing: an updated primer for clinicians in the era of antimicrobial resistance: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2023;43(4):264–78. - PubMed
-
- Oteo J, Ortega A, Bartolome R, Bou G, Conejo C, Fernandez-Martinez M, et al. . Prospective multicenter study of carbapenemase-producing Enterobacteriaceae from 83 hospitals in Spain reveals high in vitro susceptibility to colistin and meropenem. Antimicrob Agents Chemother. 2015;59(6):3406–12. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

