Correction of Griscelli Syndrome Type 2 causing mutations in the RAB27A gene with CRISPR/Cas9
- PMID: 39474038
- PMCID: PMC11518329
- DOI: 10.55730/1300-0152.2705
Correction of Griscelli Syndrome Type 2 causing mutations in the RAB27A gene with CRISPR/Cas9
Abstract
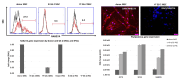
Background/aim: Griscelli Syndrome Type 2 (GS-2) is a rare, inherited immune deficiency caused by a mutation in the RAB27A gene. The current treatment consists of hematopoietic stem cell transplantation, but a lack of suitable donors warrants the development of alternative treatment strategies, including gene therapy. The development of mutation-specific clustered regularly interspaced palindromic repeats (CRISPR)/Cas9 gene editing technology has opened the way for custom-designed gene correction of patient-derived stem cells. In this study, we aimed to custom design CRISPR/Cas9 constructs and test their efficiency on homology-directed repair (HDR) on the correction of exon 3 and exon 7 mutations in the RAB27A gene of GS-2 patient-derived mesenchymal stem cells (MSCs) and induced pluripotent stem cells.
Materials and methods: We assessed RAB27A gene and protein expression using qRT-PCR, Western Blot, and immune fluorescence in GS-2 patient-derived MSCs and induced pluripotent stem cells (iPSCs). Guide RNAs (gRNAs) and donor DNAs were designed based on patient mutations in exon 3 and exon 7 using the CHOPCHOP online tool and transfected into GS-2 MSCs and iPSCs by electroporation. The cells were cultured for 2 days and then used for mutation analysis using DNA sequencing.
Results: MSCs and iPSCs from the GS-2 patients lacked RAB27A gene and protein expression. After gRNA and donor DNAs were designed and optimized, we found HDR efficiency with gRNA3.3 (10% efficiency) and gRNA7.3 (27% efficiency) for MSCs but lower efficiency in iPSCs (<5%). However, transfection of both MSCs and iPSCs resulted in massive cell death, loss of colony formation, and spontaneous differentiation.
Conclusion: The use of CRISPR/Cas9 to genetically correct MSCs and iPSCs from GS-2 patients with different mutations through HDR is feasible but requires optimization of the procedure to reduce cell death and improve stem cell function before clinical application.
Keywords: CRISPR/Cas9; electroporation; homology-directed repair; nonhomologous end joining.
© TÜBİTAK.
Conflict of interest statement
Conflict of interest: The authors declare no conflicts of interest.
Figures




References
-
- Benati D, Leung A, Perdigao P, Toulis V, van der Spuy J, et al. Induced pluripotent stem cells and genome-editing tools in determining gene function and therapy for inherited retinal disorders. International Journal of Molecular Sciences. 2022;23(23):15276. doi: 10.3390/ijms232315276. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
