Association Between Clinical Measures of Depth of Sedation and Multimodal Cerebral Physiology in Acute Traumatic Neural Injury
- PMID: 39474098
- PMCID: PMC11513567
- DOI: 10.1089/neur.2024.0090
Association Between Clinical Measures of Depth of Sedation and Multimodal Cerebral Physiology in Acute Traumatic Neural Injury
Abstract
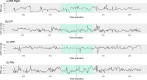
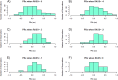
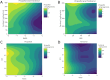
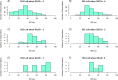
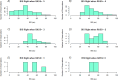
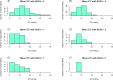
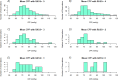
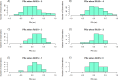
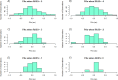
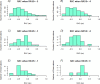
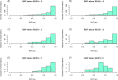
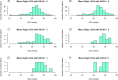
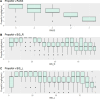
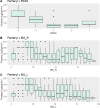
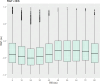
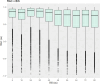
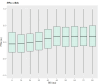
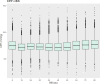
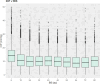
Neurointensive care primarily focuses on secondary injury reduction, utilizing a variety of guideline-based approaches (including administration of high-dose sedation) to reduce the injured state. However, titration of sedation is currently based on the Richmond Agitation Sedation Scale (RASS), a subjective clinical grading score of a patient's response to external physical stimuli, and not an objective measure. Therefore, it is likely that there exists substantial variation in objective sedation depth for a given clinical grade in these patients, leading to undesired sedation depths and cerebral physiological consequences. Improper sedation can impede cerebral autoregulation, emphasizing the critical need for optimal sedation in traumatic brain injury (TBI) patients. This study evaluates the relationship between RASS to an objective measure of depth of sedation (bispectral index, BIS) and cerebral physiological measures. Fifty-nine patients were assessed using Jonckheere-Terpstra testing to compare various key physiologies with RASS. RASS (-5 through 0 categories) showed no statistically significant relationship between BIS and cerebral physiological parameters, after adjusting for multiple comparisons. Furthermore, it is crucial to note that within each RASS value, the distribution of the physiological measures all had high variability. As an exemplar, for RASS values of -5 and -4, BIS ranged from near 0 (burst suppression levels) up to over 80 (near awake states). BIS and other cerebral physiologies displayed substantial variation across each RASS category. This suggests that RASS as a means to titrate sedative medication for the goal of neuroprotection is insufficient. More momentary, individualized determination of sedation depth is required for TBI patients.
Keywords: Bispectral Index; Cerebral Autoregulation; Critical Care; Depth of Sedation; Intracranial Pressure; Pressure Reactivity Index; RASS; Richmond Agitation Sedation Scale; Traumatic Brain Injury.
© The Author(s) 2024. Published by Mary Ann Liebert, Inc.
Figures















References
-
- Carney N, Totten AM, O’Reilly C, et al. Guidelines for the management of severe traumatic brain injury. Neurosurg 2016:244.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
