Transition Metal/Lanthanide-Nitrogen Double Bonds Co-stabilized in a Carbon Cage
- PMID: 39474411
- PMCID: PMC11503684
- DOI: 10.1021/prechem.3c00123
Transition Metal/Lanthanide-Nitrogen Double Bonds Co-stabilized in a Carbon Cage
Abstract
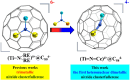
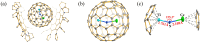
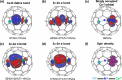
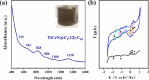
Metal-nitrogen double bonds have been commonly reported for conventional metal complexes, but the coexistence of both transition metal-nitrogen and lanthanide-nitrogen double bonds bridged by nitrogen within one compound has never been reported. Herein, by encapsulating a ternary transition metal-lanthanide heteronuclear dimetallic nitride into a C84 fullerene cage, transition metal-nitrogen and lanthanide-nitrogen double bonds are costabilized simultaneously within the as-formed clusterfullerene TiCeN@C1(12)-C84, which is a representative heteronuclear dimetallic nitride clusterfullerene. Its molecular structure was unambiguously determined by single-crystal X-ray diffraction, revealing a slightly bent μ2-bridged nitride cluster with short Ti-N (1.761 Å) and Ce-N (2.109 Å) bond lengths, which are comparable to the corresponding Ti=N and Ce=N double bonds of reported metal complexes and consistent with the theoretically predicted values, confirming their coexistence within TiCeN@C1(12)-C84. Density functional theory (DFT) calculations unveil three-center two-electron (3c-2e) bonds delocalized over the entire TiCeN cluster, which are responsible for costabilization of Ti=N and Ce=N double bonds. An electronic configuration of Ti4+Ce3+N3-@C84 4- is proposed featuring an intramolecular four-electron transfer, drastically different from the analogous actinide dimetallic nitride clusterfullerene (U2)9+N3-@C80 6- and trimetallic nitride clusterfullerene (Sc2)6+Ti3+N3-@C80 6-, indicating the peculiarity of 4-fold negatively charged fullerene cage in stabilizing the heteronuclear dimetallic nitride cluster.
© 2024 The Authors. Co-published by University of Science and Technology of China and American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Nugent W. A.; Mayer J. M.. Metal-Ligand Multiple Bonds; Wiley-Interscience: New York, 1988.
-
- Suarez A. I. O.; Lyaskovskyy V.; Reek J. N. H.; van der Vlugt J. I.; de Bruin B. Complexes with Nitrogen-Centered Radical Ligands: Classification, Spectroscopic Features, Reactivity, and Catalytic Applications. Angew. Chem., Int. Ed. 2013, 52 (48), 12510–12529. 10.1002/anie.201301487. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
