Extracellular vesicles from human breast cancer-resistant cells promote acquired drug resistance and pro-inflammatory macrophage response
- PMID: 39474419
- PMCID: PMC11518763
- DOI: 10.3389/fimmu.2024.1468229
Extracellular vesicles from human breast cancer-resistant cells promote acquired drug resistance and pro-inflammatory macrophage response
Erratum in
-
Corrigendum: Extracellular vesicles from human breast cancer-resistant cells promote acquired drug resistance and pro-inflammatory macrophage response.Front Immunol. 2025 Apr 16;16:1595885. doi: 10.3389/fimmu.2025.1595885. eCollection 2025. Front Immunol. 2025. PMID: 40308613 Free PMC article.
Abstract
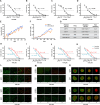
Introduction: Breast cancer is a significant public health problem around the world, ranking first in deaths due to cancer in females. The therapy to fight breast cancer involves different methods, including conventional chemotherapy. However, the acquired resistance that tumors develop during the treatment is still a central cause of cancer-associated deaths. One mechanism that induces drug resistance is cell communication via extracellular vesicles (EVs), which can carry efflux transporters and miRNA that increase sensitive cells' survivability to chemotherapy.
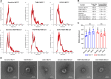
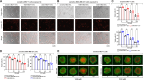
Methods: Our study investigates the transcription changes modulated by EVs from tamoxifen- and doxorubicin-resistant breast cancer cells in sensitive cells and how these changes may induce acquired drug resistance, inhibit apoptosis, and increase survivability in the sensitive cells. Additionally, we exposed human macrophages to resistant EVs to understand the influence of EVs on immune responses.
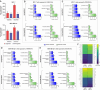
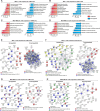
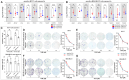
Results: Our results suggest that the acquired drug resistance is associated with the ability of resistant EVs to upregulate several transporter classes, which are directly related to the increase of cell viability and survival of sensitive cells exposed to EVs before a low-dose drug treatment. In addition, we show evidence that resistant EVs may downregulate immune system factors to evade detection and block cell death by apoptosis in sensitive breast cancer cells. Our data also reveals that human macrophages in contact with resistant EVs trigger a pro-inflammatory cytokine secretion profile, an effect that may be helpful for future immunotherapy studies.
Discussion: These findings are the first transcriptome-wide analysis of cells exposed to resistant EVs, supporting that resistant EVs are associated with the acquired drug resistance process during chemotherapy by modulating different aspects of sensitive cancer cells that coffer the chemoresistance.
Keywords: chemoresistance; doxorubicin; immunomodulation; membrane transporters; tamoxifen.
Copyright © 2024 Santos, Rezende, Piraine, Oliveira, Ferreira, Carvalho, Calado, Pellegrini and Almeida.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

