Association between Exposure to Metals during Pregnancy, Childhood Gut Microbiome, and Risk of Intestinal Inflammation in Late Childhood
- PMID: 39474439
- PMCID: PMC11501044
- DOI: 10.1021/envhealth.4c00125
Association between Exposure to Metals during Pregnancy, Childhood Gut Microbiome, and Risk of Intestinal Inflammation in Late Childhood
Abstract
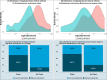
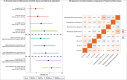
Alterations to the gut microbiome and exposure to metals during pregnancy have been suggested to impact inflammatory bowel disease. Nonetheless, how prenatal exposure to metals eventually results in long-term effects on the gut microbiome, leading to subclinical intestinal inflammation, particularly during late childhood, has not been studied. It is also unknown whether such an interactive effect drives a specific subgroup of children toward elevated susceptibility to intestinal inflammation. We used an amalgamation of machine-learning techniques with a regression-based framework to explore if children with distinct sets of gut microbes and certain patterns of exposure to metals during pregnancy (metal-microbial clique signature) had a higher likelihood of intestinal inflammation, measured based on fecal calprotectin (FC) in late childhood. We obtained samples from a well-characterized longitudinal birth cohort from Mexico City (n = 108), Mexico. In the second and third trimesters of pregnancy, 11 metals were measured in whole blood. Gut microbial abundances and FC were measured in stool samples from children 9-11 years of age. Elevated FC was defined as having FC above 100 μg/g of stool. We identified subgroups of children in whom microbial and metal-microbial clique signatures were associated with elevated FC (false discovery rate (FDR) < 0.05). In particular, we found two metal-microbial clique signatures significantly associated with elevated FC: (1) low cesium (Cs) and copper (Cu) in the third trimester and low relative abundance of Eubacterium ventriosum (OR [95%CI]: 10.27 [3.57,29.52], FDR < 0.001) and (2) low Cu in the third trimester and high relative abundances of Roseburia inulinivorans and Ruminococcus torques (OR [95%CI]: 7.21 [1.81,28.77], FDR < 0.05). This exploratory study demonstrates that children with specific gut microbes and specific exposure patterns to metals during pregnancy may have higher fecal calprotectin levels in late childhood, denoting an elevated risk of intestinal inflammation.
© 2024 The Authors. Co-published by Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, and American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): The corresponding author confirms on behalf of all authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated. V.M., C.G., L.T., L.A.T.-O., J.E., M.P., J.K.G., I.P., M.M.T.-R., R.O.W., and S.E. report no conflicts of interest. M. Agrawal has consulted for Douglas Pharmaceuticals. J.J.F. is on the Scientific Advisory Board of Vedanta Biosciences. M. Arora is an employee and equity holder of Linus Biotechnology Inc., a start-up company of Mount Sinai Health System that develops tools for the detection of autism spectrum disorder and related conditions. J.-F.C. reports receiving research grants from AbbVie, Janssen Pharmaceuticals and Takeda; receiving payment for lectures from AbbVie, Amgen, Allergan Inc., Ferring Pharmaceuticals, Shire, and Takeda; receiving consulting fees from AbbVie, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb, Celgene Corporation, Eli Lilly, Ferring Pharmaceuticals, Galmed Research, Glaxo Smith Kline, Geneva, Iterative Scopes, Janssen Pharmaceuticals, Kaleido Biosciences, Landos, Otsuka, Pfizer, Prometheus, Sanofi, Takeda, TiGenix; and holding stock options in Intestinal Biotech Development.
Figures

References
-
- Turpin W.; Dong M.; Sasson G.; Raygoza Garay J. A.; Espin-Garcia O.; Lee S.-H.; Neustaeter A.; Smith M. I.; Leibovitzh H.; Guttman D. S.; Goethel A.; Griffiths A. M.; Huynh H. Q.; Dieleman L. A.; Panaccione R.; Steinhart A. H.; Silverberg M. S.; Aumais G.; Jacobson K.; Mack D.; Murthy S. K.; Marshall J. K.; Bernstein C. N.; Abreu M. T.; Moayyedi P.; Paterson A. D.; Xu W.; Croitoru K. Mediterranean-Like Dietary Pattern Associations With Gut Microbiome Composition and Subclinical Gastrointestinal Inflammation. Gastroenterology 2022, 163 (3), 685–698. 10.1053/j.gastro.2022.05.037. - DOI - PubMed
-
- Raygoza Garay J. A.; Turpin W.; Lee S.-H.; Smith M. I.; Goethel A.; Griffiths A. M.; Moayyedi P.; Espin-Garcia O.; Abreu M.; Aumais G. L.; Bernstein C. N.; Biron I. A.; Cino M.; Deslandres C.; Dotan I.; El-Matary W.; Feagan B.; Guttman D. S.; Huynh H.; Dieleman L. A.; Hyams J. S.; Jacobson K.; Mack D.; Marshall J. K.; Otley A.; Panaccione R.; Ropeleski M.; Silverberg M. S.; Steinhart A. H.; Turner D.; Yerushalmi B.; Paterson A. D.; Xu W.; Croitoru K. Gut Microbiome Composition Is Associated With Future Onset of Crohn’s Disease in Healthy First-Degree Relatives. Gastroenterology 2023, 165 (3), 670–681. 10.1053/j.gastro.2023.05.032. - DOI - PubMed
-
- Torres J.; Hu J.; Seki A.; Eisele C.; Nair N.; Huang R.; Tarassishin L.; Jharap B.; Cote-Daigneault J.; Mao Q.; Mogno I.; Britton G. J.; Uzzan M.; Chen C.-L.; Kornbluth A.; George J.; Legnani P.; Maser E.; Loudon H.; Stone J.; Dubinsky M.; Faith J. J.; Clemente J. C.; Mehandru S.; Colombel J.-F.; Peter I. Infants Born to Mothers with IBD Present with Altered Gut Microbiome That Transfers Abnormalities of the Adaptive Immune System to Germ-Free Mice. Gut 2020, 69 (1), 42–51. 10.1136/gutjnl-2018-317855. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
