Advances in Small-Molecule Fluorescent pH Probes for Monitoring Mitophagy
- PMID: 39474479
- PMCID: PMC11503929
- DOI: 10.1021/cbmi.3c00070
Advances in Small-Molecule Fluorescent pH Probes for Monitoring Mitophagy
Abstract
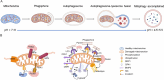
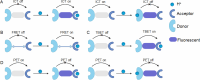
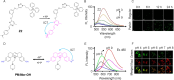
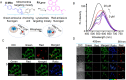
Mitochondria play a crucial role in regulating cellular energy homeostasis and cell death, making them essential organelles. Maintaining proper cellular functions relies on the removal of damaged mitochondria through a process called mitophagy. Mitophagy is associated with changes in the pH value and has implications for numerous diseases. To effectively monitor mitophagy, fluorescent probes that exhibit high selectivity and sensitivity based on pH detection have emerged as powerful tools. In this review, we present recent advancements in the monitoring of mitophagy using small-molecule fluorescence pH probes. We focus on various sensing mechanisms employed by these probes, including intramolecular charge transfer (ICT), fluorescence resonance energy transfer (FRET), through bond energy transfer (TBET), and photoelectron transfer (PET). Additionally, we discuss disease models used for studying mitophagy and summarize the design requirements for small-molecule fluorescent pH probes suitable for monitoring the mitophagy process. Lastly, we highlight the remaining challenges in this field and propose potential directions for the future development of mitophagy probes.
© 2023 The Authors. Co-published by Nanjing University and American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



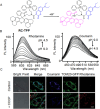

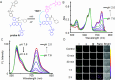

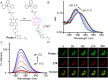
References
-
- She M.-T.; Yang J.-W.; Zheng B.-X.; Long W.; Huang X.-H.; Luo J.-R.; Chen Z.-X.; Liu A.-L.; Cai D.-P.; Wong W.-L.; et al. Design Mitochondria-Specific Fluorescent Turn-on Probes Targeting G-Quadruplexes for Live Cell Imaging and Mitophagy Monitoring Study. Chem. Eng. J. 2022, 446, 136947–136961. 10.1016/j.cej.2022.136947. - DOI
Publication types
LinkOut - more resources
Full Text Sources
