Durvalumab-Induced Immune Thrombocytopenia in Patients with Advanced Cholangiocarcinoma Undergoing Yttrium-90 Radioembolization
- PMID: 39474535
- PMCID: PMC11521497
- DOI: 10.1159/000541550
Durvalumab-Induced Immune Thrombocytopenia in Patients with Advanced Cholangiocarcinoma Undergoing Yttrium-90 Radioembolization
Abstract
Introduction: Immune thrombocytopenia (ITP) secondary to durvalumab, a programmed cell death ligand 1 inhibitor, is a rare but clinically significant immune-related adverse event. Herein, we present 2 patients with cholangiocarcinoma who developed ITP immediately post-yttrium-90 radioembolization (Y90-RE) while on durvalumab-based systemic therapy. We hypothesize that given the timing, the immunotherapy and the radioembolization combination led to this event. It is not uncommon given the approval of immunotherapy and its role in locoregional therapies, that patients are treated with a combination of systemic immunotherapy and radioembolization or other forms of radiation, thus signifying the importance of potential complications.
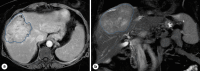
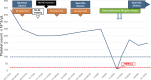
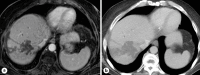
Case presentation: Two patients, a 67-year-old female and a 60-year-old man, with biopsy-proven advanced unresectable cholangiocarcinoma, received a combination of systemic therapy with durvalumab, gemcitabine, and cisplatin and subsequently Y90-RE. Both patients developed ITP following in the immediate post-Y90-RE period. All other causes of ITP were comprehensively ruled out and treatment for ITP was initiated in the form of high-dose steroid and intravenous immunoglobulins. Durvalumab was discontinued, and only gemcitabine/cisplatin-based chemotherapy was continued thereafter. Due to recurrence, one of the patients required longer courses of steroids as well as thrombopoietin receptor agonists.
Conclusion: Immunotherapy in the form of durvalumab and now pembrolizumab alongside chemotherapy is an approved first-line standard of care. Furthermore, it is not uncommon for patients to receive Y90-RE to improve patient outcomes. This report highlights the development of ITP in 2 patients who received durvalumab alongside Y90-RE. Awareness of this as a potential immune-mediated event is important to allow for close monitoring of platelet counts and for early intervention/management when this occurs.
Keywords: Cholangiocarcinoma; Durvalumab; Immune thrombocytopenia; Yttrium-90.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
P.M.K. reports grants paid to the institution by Merck, Agenus Bio, Novartis, Advanced Accelerator Applications, TerSera, and Boston Scientific; a consultancy and advisory board relationship with Elicio (scientific advisory board member/shares/stock ownership); cofounder of Precision BioSensors Inc.; consultancy/advisory board fees from Guardant Health, Natera, Foundation Medicine, Illumina, BostonGene, Merck/MSD Oncology, Tempus, Bayer, Lilly, Delcath Systems, IPBA, QED Therapeutics, Boston Healthcare Associates, Servier, Taiho Oncology, Exact Sciences, Daiichi Sankyo/AstraZeneca, Eisai, Saga Diagnostics, NeoGenomics, DoMore Diagnostics AS, and Seattle Genetics; consulting fees paid to the institution by Taiho Pharmaceutical and Ipsen; receiving travel support from AstraZeneca for presentation of an investigator-initiated trial. All other authors have no conflicts of interest to report.
Figures



References
-
- Oh DY, Ruth He A, Qin S, Chen LT, Okusaka T, Vogel A, et al. . Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8):EVIDoa2200015. - PubMed
-
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. . Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81. - PubMed
-
- Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. . Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853–65. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

