Effectiveness and Safety of Hair Growth Formulation Containing Tectona grandis L.f (Teak) Leaf Extract: A Randomized, Double-Blind, Placebo-Controlled Study on Males with Androgenic Alopecia
- PMID: 39474646
- PMCID: PMC11612263
- DOI: 10.1177/2515690X241291141
Effectiveness and Safety of Hair Growth Formulation Containing Tectona grandis L.f (Teak) Leaf Extract: A Randomized, Double-Blind, Placebo-Controlled Study on Males with Androgenic Alopecia
Abstract
Background: Androgenic alopecia (AGA) is commonly known as male patterned baldness. A high level of dihydrotestosterone (DHT) plays a significant role in AGA development. Inhibition of the enzyme steroid 5-alpha reductase (S5AR), responsible for converting testosterone into DHT, has been shown to delay the progression of AGA. Teak (Tectona grandis L.f) leaf extract exhibited a potent S5AR inhibitory activity. To prove the effectiveness and safety of teak leaf extract as a hair growth promotor, a double-blind, randomized placebo-controlled trial was conducted.
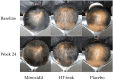
Methods: Eighty-one AGA subjects were randomly assigned to receive either a hair tonic containing 1% teak leaf extract (HT-teak), 5%minoxidil (positive control), or a placebo administered twice daily, for 24 weeks. Efficacy was assessed through target area hair count (TAHC), anagen-to-telogen ratio (A/T), hair shedding every 4 weeks, and patients' subjective assessments of hair regrowth were assessed at the end of the experiment. Data was analyzed using repeated measure ANOVA.
Results: Both the HT-teak and minoxidil groups exhibited a significant increase in TAHC and A/T, along with a decrease in hair shedding compared to baseline values. Conversely, the placebo group showed no observable signs of hair regrowth. Furthermore, the HT-teak group reported the highest satisfaction scores, and there were no indications of skin irritation or systemic effects on sexual dysfunction and palpitation after 24 weeks of HT-teak application.
Conclusion: Teak leaf extract, as incorporated in HT-teak, demonstrates potential as an alternative mild hair growth promoter for individuals with AGA, offering both efficacy and safety.
Trial registration: This study was retrospectively registered on International Standard Randomised Controlled Trial Number (ISRCTN.com); ISRCTN24541842 (registered on January 8, 2024).
Keywords: 5-alpha reductase inhibitor; androgenic alopecia; clinical trial; hair growth; teak; tectona grandis l.f.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Saartok T, Dahlberg E, Gustafsson J-A. Relative binding affinity of anabolic-androgenic steroids: Comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin. Endocrinology. 1984;114(6):2100-2106. doi:10.1210/endo-114-6-2100 - DOI - PubMed
-
- Schweiger ES, Boychenko O, Bernstein RM. Update on the pathogenesis, genetics and medical treatment of patterned hair loss. Journal of Drugs in Dermatology: JDD. 2010;9(11):1412-1419. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

