Using QALYs as an Outcome for Assessing Global Prediction Accuracy in Diabetes Simulation Models
- PMID: 39474832
- PMCID: PMC11645849
- DOI: 10.1177/0272989X241285866
Using QALYs as an Outcome for Assessing Global Prediction Accuracy in Diabetes Simulation Models
Abstract
Objectives: (1) To demonstrate the use of quality-adjusted life-years (QALYs) as an outcome measure for comparing performance between simulation models and identifying the most accurate model for economic evaluation and health technology assessment. QALYs relate directly to decision making and combine mortality and diverse clinical events into a single measure using evidence-based weights that reflect population preferences. (2) To explore the usefulness of Q2, the proportional reduction in error, as a model performance metric and compare it with other metrics: mean squared error (MSE), mean absolute error, bias (mean residual), and R2.
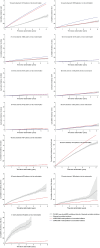
Methods: We simulated all EXSCEL trial participants (N = 14,729) using the UK Prospective Diabetes Study Outcomes Model software versions 1 (UKPDS-OM1) and 2 (UKPDS-OM2). The EXSCEL trial compared once-weekly exenatide with placebo (median 3.2-y follow-up). Default UKPDS-OM2 utilities were used to estimate undiscounted QALYs over the trial period based on the observed events and survival. These were compared with the QALYs predicted by UKPDS-OM1/2 for the same period.
Results: UKPDS-OM2 predicted patients' QALYs more accurately than UKPDS-OM1 did (MSE: 0.210 v. 0.253; Q2: 0.822 v. 0.786). UKPDS-OM2 underestimated QALYs by an average of 0.127 versus 0.150 for UKPDS-OM1. UKPDS-OM2 predictions were more accurate for mortality, myocardial infarction, and stroke, whereas UKPDS-OM1 better predicted blindness and heart disease. Q2 facilitated comparisons between subgroups and (unlike R2) was lower for biased predictors.
Conclusions: Q2 for QALYs was useful for comparing global prediction accuracy (across all clinical events) of diabetes models. It could be used for model registries, choosing between simulation models for economic evaluation and evaluating the impact of recalibration. Similar methods could be used in other disease areas.
Highlights: Diabetes simulation models are currently validated by examining their ability to predict the incidence of individual events (e.g., myocardial infarction, stroke, amputation) or composite events (e.g., first major adverse cardiovascular event).We introduce Q2, the proportional reduction in error, as a measure that may be useful for evaluating and comparing the prediction accuracy of econometric or simulation models.We propose using the Q2 or mean squared error for QALYs as global measures of model prediction accuracy when comparing diabetes models' performance for health technology assessment; these can be used to select the most accurate simulation model for economic evaluation and to evaluate the impact of model recalibration in diabetes or other conditions.
Keywords: microsimulation; model performance; patient-level simulation; quality-adjusted life-years; risk modeling; type 2 diabetes mellitus.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors have completed the ICMJE uniform disclosure form.
Figures




References
-
- Mount Hood Diabetes Challenge Network. Economics, simulation modelling & diabetes. Available from: https://www.mthooddiabeteschallenge.com/ [Accessed 15 November, 2021].
-
- Hayes AJ, Leal J, Gray AM, Holman RR, Clarke PM. UKPDS Outcomes Model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56:1925–33. DOI: 10.1007/s00125-013-2940-y - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

