The post-translational modification O-GlcNAc is a sensor and regulator of metabolism
- PMID: 39474868
- PMCID: PMC11523104
- DOI: 10.1098/rsob.240209
The post-translational modification O-GlcNAc is a sensor and regulator of metabolism
Abstract
Cells must rapidly adapt to changes in nutrient conditions through responsive signalling cascades to maintain homeostasis. One of these adaptive pathways results in the post-translational modification of proteins by O-GlcNAc. O-GlcNAc modifies thousands of nuclear and cytoplasmic proteins in response to nutrient availability through the hexosamine biosynthetic pathway. O-GlcNAc is highly dynamic and can be added and removed from proteins multiple times throughout their life cycle, setting it up to be an ideal regulator of cellular processes in response to metabolic changes. Here, we describe the link between cellular metabolism and O-GlcNAc, and we explore O-GlcNAc's role in regulating cellular processes in response to nutrient levels. Specifically, we discuss the mechanisms of elevated O-GlcNAc levels in contributing to diabetes and cancer, as well as the role of decreased O-GlcNAc levels in neurodegeneration. These studies form a foundational understanding of aberrant O-GlcNAc in human disease and provide an opportunity to further improve disease identification and treatment.
Keywords: O-GlcNAc; metabolism; modifications; post-translational; regulators; sensor.
Conflict of interest statement
We declare we have no competing interests.
Figures

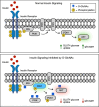
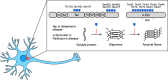
References
-
- Shafi R, Iyer SP, Ellies LG, O’Donnell N, Marek KW, Chui D, Hart GW, Marth JD. 2000. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc. Natl Acad. Sci. USA 97, 5735–5739. ( 10.1073/pnas.100471497) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
