PKM2-Driven Lactate Overproduction Triggers Endothelial-To-Mesenchymal Transition in Ischemic Flap via Mediating TWIST1 Lactylation
- PMID: 39474980
- PMCID: PMC11653614
- DOI: 10.1002/advs.202406184
PKM2-Driven Lactate Overproduction Triggers Endothelial-To-Mesenchymal Transition in Ischemic Flap via Mediating TWIST1 Lactylation
Abstract
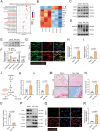
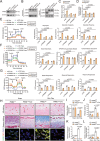
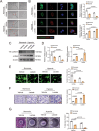
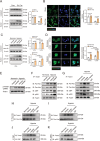
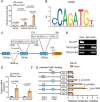
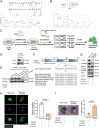
The accumulation of lactate is a rising risk factor for patients after flap transplantation. Endothelial-to-mesenchymal transition (EndoMT) plays a critical role in skin fibrosis. Nevertheless, whether lactate overproduction directly contributes to flap necrosis and its mechanism remain unknown. The current study reveals that skin flap mice exhibit enhanced PKM2 and fibrotic response. Endothelial-specific deletion of PKM2 attenuates flap necrosis and ameliorates flap fibrosis in mice. Administration of lactate or overexpressing PKM2 promotes dysfunction of endothelial cells and stimulates mesenchymal-like phenotype following hypoxia. Mechanistically, glycolytic-lactate induces a correlation between Twist1 and p300/CBP, leading to lactylation of Twist1 lysine 150 (K150la). The increase in K150la promotes Twist1 phosphorylation and nuclear translocation and further regulates the transcription of TGFB1, hence inducing fibrosis phenotype. Genetically deletion of endothelial-specific PKM2 in mice diminishes lactate accumulation and Twist1 lactylation, then attenuates EndoMT-associated fibrosis following flap ischemia. The serum lactate levels of flap transplantation patients are elevated and exhibit predictive value for prognosis. This findings suggested a novel role of PKM2-derived lactate in mediating Twist1 lactylation and exacerbates flap fibrosis and ischemia. Inhibition of glycolytic-lactate and Twist1 lactylation reduces flap necrosis and fibrotic response might become a potential therapeutic strategy for flap ischemia.
Keywords: Pyruvate kinase M2; endothelial‐to‐mesenchymal transition; lactate; lactylation; random‐pattern skin flap.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
