An evaluation of vilobelimab (anti-C5a) as a cost-effective option to treat severely ill mechanically ventilated patients with COVID-19
- PMID: 39475087
- PMCID: PMC12039489
- DOI: 10.1093/ajhp/zxae318
An evaluation of vilobelimab (anti-C5a) as a cost-effective option to treat severely ill mechanically ventilated patients with COVID-19
Abstract
Purpose: COVID-19 patients in intensive care units (ICUs) requiring invasive mechanical ventilation (IMV) have few available treatment options. PANAMO, a multicenter, double-blind, randomized, placebo-controlled phase 3 study of vilobelimab, which blocks the inflammatory process caused by complement component 5a, demonstrated a significant mortality benefit at 28 and 60 days in these patients. A cost-effectiveness analysis was conducted to assess the incremental cost per quality-adjusted life-year (QALY).
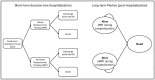
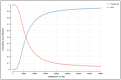
Methods: A Markov model was used to estimate QALYs and the incremental cost-effectiveness ratio (ICER) of vilobelimab plus standard of care (SOC) versus SOC alone. The model simulated progression from severe COVID-19 to survival or death over a lifetime horizon. Outcomes data (COVID-19 all-cause mortality and renal replacement therapy) were incorporated from the PANAMO trial. COVID-19 mortality estimates were based on Centers for Disease Control and Prevention age-specific survival data. Utility values and hospital costs came from the literature. Vilobelimab cost was obtained from RED BOOK Online.
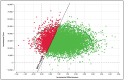
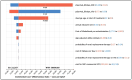
Results: For COVID-19 ICU patients, total costs of care were $103,414 (SOC) and $132,247 (SOC plus vilobelimab), respectively, resulting in an incremental cost of $28,833. SOC provided 6.70 QALYs versus 7.99 QALYs for vilobelimab, an additional 1.29 QALYs. The ICER for vilobelimab plus SOC versus SOC alone was $22,287/QALY. Probabilistic sensitivity analysis demonstrated the robustness of the cost-effectiveness result as vilobelimab plus SOC was favored at a willingness-to-pay threshold of $50,000 in over 81% of iterations.
Conclusion: Vilobelimab provides a cost-effective option to treat ICU patients with severe COVID-19 receiving IMV compared to SOC, at well below the commonly accepted $50,000 US willingness-to-pay threshold.
Keywords: COVID-19; QALY; cost-effectiveness analysis; quality adjusted life years; treatment effectiveness; vilobelimab.
© American Society of Health-System Pharmacists 2024.
Figures
References
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC.. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782-793. doi: https://doi.org/10.1001/jama.2020.12839 - DOI - PubMed
-
- de Bruin S, Bos LD, van Roon MA, et al.Clinical features and prognostic factors in Covid-19: a prospective cohort study. EBioMedicine. 2021;67:103378. doi: https://doi.org/10.1016/j.ebiom.2021.103378 - DOI - PMC - PubMed
-
- Writing Committee for the REMAP-CAP Investigators. Long-term (180-day) outcomes in critically ill patients with COVID-19 in the REMAP-CAP randomized clinical trial. JAMA. 2023;329(1):39-51. doi: https://doi.org/10.1001/jama.2022.23257 - DOI - PMC - PubMed
-
- Docherty AB, Harrison EM, Green CA, et al.Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: https://doi.org/10.1136/bmj.m1985 - DOI - PMC - PubMed
-
- Afzali B, Noris M, Lambrecht BN, Kemper C.. The state of complement in COVID-19. Nat Rev Immunol. 2022;22(2):77-84. doi: https://doi.org/10.1038/s41577-021-00665-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

