Improving Neck and Jawline Aesthetics With OnabotulinumtoxinA by Minimizing Platysma Muscle Contraction Effects: Efficacy and Safety Results in a Phase 3 Randomized, Placebo-Controlled Study
- PMID: 39475141
- PMCID: PMC11736717
- DOI: 10.1093/asj/sjae220
Improving Neck and Jawline Aesthetics With OnabotulinumtoxinA by Minimizing Platysma Muscle Contraction Effects: Efficacy and Safety Results in a Phase 3 Randomized, Placebo-Controlled Study
Abstract
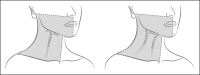
Background: Platysma prominence (PP) describes the noticeable appearance of the platysma muscle upon contraction, causing a less defined jawline contour and vertical neck bands.
Objectives: The objective of this study was to assess the safety and efficacy of onabotulinumtoxinA for improvement of PP in adults.
Methods: Participants with moderate to severe (Grade 3 to 4) PP at maximum contraction received onabotulinumtoxinA or placebo on Day 1 and were monitored for 120 days. OnabotulinumtoxinA dosage (26, 31, or 36 U) was customized based on baseline PP severity on each side of the neck.
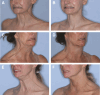
Results: Efficacy analyses were conducted in the intent-to-treat (ITT) population (all randomized participants), and modified ITT population (mITT; psychosocially impacted by PP appearance). Results from ITT and mITT populations were comparable. As assessed by investigators, 76.7% of onabotulinumtoxinA mITT participants achieved ≥1-grade improvement vs 21.2% in the placebo group, and 41.0% vs 2.2% (P < .0001) achieved ≥2-grade improvement at Day 14. As assessed by participants, 79.9% of onabotulinumtoxinA mITT participants vs 21.8% in the placebo group and 40.8% vs 3.9% (P < .0001) achieved ≥1- or ≥2-grade improvement, respectively, at Day 14. OnabotulinumtoxinA responder rates remained higher than placebo through Day 120, gradually declining over time. OnabotulinumtoxinA participants reported significantly higher satisfaction with treatment effect, less bother from jawline and vertical neck bands, and lower psychosocial impact from PP than placebo at Day 14 (P < .0001). OnabotulinumtoxinA effectively improved self-perceived jawline definition and was well tolerated.
Conclusions: OnabotulinumtoxinA was well tolerated and effective at improving moderate to severe PP, including neck bands and jawline definition.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Aesthetic Society.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

