Epidermal growth factor receptor signaling governs the host inflammatory response to invasive aspergillosis
- PMID: 39475281
- PMCID: PMC11633379
- DOI: 10.1128/mbio.02671-24
Epidermal growth factor receptor signaling governs the host inflammatory response to invasive aspergillosis
Abstract
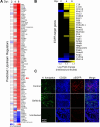
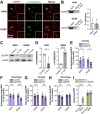
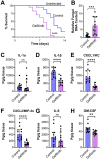
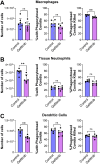
The epidermal growth factor receptor (EGFR) has been identified as an epithelial cell receptor for Mucorales fungi and Candida albicans. Blocking EGFR with small molecule inhibitors reduces disease severity in mouse models of mucormycosis and oropharyngeal candidiasis. In contrast, cases of invasive aspergillosis have been reported in cancer patients who were treated with EGFR inhibitors, suggesting that EGFR signaling may play a protective role in the host defense against this infection. Here, we analyzed transcriptomic data from the lungs of mice with invasive aspergillosis and found evidence that Aspergillus fumigatus infection activates multiple genes that are predicted to function in the EGFR signaling pathway. We also found that A. fumigatus infection activates EGFR in both a human small-airway epithelial (HSAE) cell line and in the lungs of immunosuppressed mice. EGFR signaling in HSAE cells is required for maximal endocytosis of A. fumigatus and for fungal-induced proinflammatory cytokine and chemokine production. In a corticosteroid immunosuppressed mouse model of invasive pulmonary aspergillosis, inhibition of EGFR with gefitinib decreased whole-lung cytokine and chemokine levels and reduced accumulation of phagocytes in the lung, leading to a decrease in fungal killing, an increase in pulmonary fungal burden, and accelerated mortality. Thus, EGFR signaling is required for pulmonary epithelial cells to orchestrate the host innate immune defense against invasive aspergillosis in immunosuppressed hosts.IMPORTANCEWhen A. fumigatus infects the lungs, it invades epithelial cells that line the airways. During this process, the fungus interacts with epithelial cell receptors. This interaction stimulates epithelial cells to endocytose the fungus. It also induces these cells to secrete proinflammatory cytokines and chemokines that recruit phagocytes to the site of infection where they can kill the fungus. Here, we show that in small-airway epithelial cells, the EGFR acts as a sensor for A. fumigatus that triggers the production of chemokines in response to fungal infection. In corticosteroid-immunosuppressed mice, blocking EGFR with the kinase inhibitor gefitinib reduces chemokine production in the lungs. This leads to decreased accumulation of neutrophils and dendritic cells in the lungs, reduced A. fumigatus killing, and increased mortality. These results provide a potential explanation as to why some cancer patients who are treated with EGFR inhibitors develop invasive aspergillosis.
Keywords: Aspergillus fumigatus; alveolar epithelial cell; chemokine; cytokine; endocytosis; epidermal growth factor receptor; small-airway epithelial cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Update of
-
Epidermal Growth Factor Receptor Signaling Governs the Host Inflammatory Response to Invasive Aspergillosis.bioRxiv [Preprint]. 2024 Sep 10:2024.09.10.612305. doi: 10.1101/2024.09.10.612305. bioRxiv. 2024. Update in: mBio. 2024 Dec 11;15(12):e0267124. doi: 10.1128/mbio.02671-24. PMID: 39314401 Free PMC article. Updated. Preprint.
References
-
- Maertens JA, Rahav G, Lee D-G, Ponce-de-León A, Ramírez Sánchez IC, Klimko N, Sonet A, Haider S, Diego Vélez J, Raad I, Koh L-P, Karthaus M, Zhou J, Ben-Ami R, Motyl MR, Han S, Grandhi A, Waskin H, study investigators . 2021. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: a phase 3, randomised, controlled, non-inferiority trial. Lancet 397:499–509. doi:10.1016/S0140-6736(21)00219-1 - DOI - PubMed
-
- Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, Bow EJ, Rahav G, Neofytos D, Aoun M, et al. . 2016. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. The Lancet 387:760–769. doi:10.1016/S0140-6736(15)01159-9 - DOI - PubMed
-
- Resendiz-Sharpe A, Mercier T, Lestrade PPA, van der Beek MT, von dem Borne PA, Cornelissen JJ, De Kort E, Rijnders BJA, Schauwvlieghe AFAD, Verweij PE, Maertens J, Lagrou K. 2019. Prevalence of voriconazole-resistant invasive aspergillosis and its impact on mortality in haematology patients. J Antimicrob Chemother 74:2759–2766. doi:10.1093/jac/dkz258 - DOI - PubMed
-
- Bertuzzi M, Schrettl M, Alcazar-Fuoli L, Cairns TC, Muñoz A, Walker LA, Herbst S, Safari M, Cheverton AM, Chen D, Liu H, Saijo S, Fedorova ND, Armstrong-James D, Munro CA, Read ND, Filler SG, Espeso EA, Nierman WC, Haas H, Bignell EM. 2014. The pH-responsive PacC transcription factor of Aspergillus fumigatus governs epithelial entry and tissue invasion during pulmonary aspergillosis. PLoS Pathog 10:e1004413. doi:10.1371/journal.ppat.1004413 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- U19AI110820/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- U19 AI110820/AI/NIAID NIH HHS/United States
- R01AI141360/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI162802/AI/NIAID NIH HHS/United States
- R01AI162802/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
