Association of Tumor Mutational Burden and PD-L1 with the Efficacy of Pembrolizumab with or without Chemotherapy versus Chemotherapy in Advanced Urothelial Carcinoma
- PMID: 39475359
- PMCID: PMC11609623
- DOI: 10.1158/1078-0432.CCR-23-3518
Association of Tumor Mutational Burden and PD-L1 with the Efficacy of Pembrolizumab with or without Chemotherapy versus Chemotherapy in Advanced Urothelial Carcinoma
Abstract
Purpose: The three-arm, phase III KEYNOTE-361 study did not meet its dual primary endpoints of progression-free survival (PFS) or overall survival (OS) with first-line pembrolizumab plus chemotherapy versus chemotherapy in advanced urothelial carcinoma. This prespecified exploratory analysis assessed the association of tumor mutational burden (TMB) and PD-L1 combined positive score (CPS) with clinical outcomes.
Patients and methods: TMB and PD-L1 CPS were determined via whole-exome sequencing and PD-L1 IHC 22C3 pharmDx, respectively. The association was evaluated in each treatment arm using logistic regression [objective response rate (ORR)] and Cox proportional hazards regression models (PFS and OS); one-sided (pembrolizumab monotherapy; pembrolizumab plus chemotherapy) and two-sided (chemotherapy) nominal P values were calculated. Significance was prespecified at α = 0.05 without multiplicity adjustment. Efficacy was evaluated by prespecified cutoffs of 175 mutations/exome (TMB) and CPS 10 (PD-L1).
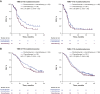
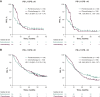
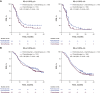
Results: Of the 993 treated patients, 820 (82.6%) and 993 (100%) had evaluable TMB and CPS data, respectively. Continuous TMB was positively associated with ORR, PFS, and OS for pembrolizumab monotherapy (one-sided P < 0.001, P < 0.001, and P = 0.007, respectively); PFS and OS for pembrolizumab plus chemotherapy (one-sided P = 0.007 and P = 0.010, respectively); and OS for chemotherapy alone (two-sided P = 0.040). Continuous PD-L1 CPS showed evidence of anticipated association with ORR and PFS for pembrolizumab monotherapy. The subgroup with TMB ≥175 mutations/exome and PD-L1 CPS ≥10 had the highest PFS and OS improvements with pembrolizumab alone or with chemotherapy versus chemotherapy alone.
Conclusions: These data suggest that TMB may be predictive of the response to pembrolizumab alone or with chemotherapy in advanced urothelial carcinoma.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A. Fléchon reports personal fees and other support from MSD, Astellas Pharma, Merck & Co., Inc., Rahway, NJ, Bristol Myers Squibb, and Janssen and personal fees from Gilead outside the submitted work. R. Morales-Barrera reports serving in an advisory role for MSD, Pfizer, Merck & Co., Inc., Rahway, NJ, Janssen, and Astellas Pharma; received honoraria or travel expenses from Roche, Sanofi Aventis, Astellas, Janssen, MSD, Bayer, Merck & Co., Inc., Rahway, NJ, and Pfizer. T. Powles reports grants from AstraZeneca, Roche, Bristol Myers Squibb, Exelixis, Ipsen, MSD, Novartis, Pfizer, Seattle Genetics, Merck Serono, Astellas Pharma, Johnson & Johnson, and Eisai outside the submitted work. A. Alva reports grants from Merck & Co., Inc., Rahway, NJ, and V Foundation during the conduct of the study. Y. Loriot reports grants, personal fees, nonfinancial support, and other support from MSD during the conduct of the study, as well as personal fees, nonfinancial support, and other support from Janssen, Pfizer, Merck KGaA, Astellas Pharma, Gilead, Bristol Myers Squibb, and Roche; and other support from Incyte, Exelixis, and Taiho outside the submitted work. A. Rodriguez-Vida reports personal fees from Bristol Myers Squibb, Roche, Merck & Co., Inc., Rahway, NJ, Pfizer, Janssen, Astellas Pharma, Sanofi, Bayer, and AstraZeneca during the conduct of the study. S. Oudard reports personal fees from Sanofi, MSD, Ipsen, Novartis, Bayer, Janssen, and Astellas Pharma during the conduct of the study. C. Vulsteke reports grants from MSD and personal fees from MSD, Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, GSK, Janssen-Cilag, LEO Pharma, Merck & Co., Inc., Rahway, NJ, and Pfizer outside the submitted work. R. Mamtani reports grants from Merck & Co., Inc., Rahway, NJ, and Astellas Pharma and personal fees from Seagen and Bristol Myers Squibb outside the submitted work. E.Y. Yu reports grants and personal fees from Merck & Co., Inc., Rahway, NJ, during the conduct of the study, as well as personal fees from Janssen, Advanced Accelerator Applications, Aadi Bioscience, Bristol Myers Squibb, and Loxo; grants and personal fees from Bayer, Oncternal, and Lantheus; and grants from Dendreon, Seagen, Blue Earth, Tyra, and Daiichi Sankyo outside the submitted work. A. Montesa Pino reports personal fees from Bristol Myers Squibb, Astellas Pharma, AstraZeneca, Novartis, MSD, Janssen, Bayer, and Advanced Accelerator Applications and personal fees and nonfinancial support from Pfizer, Merck & Co., Inc., Rahway, NJ, and Ipsen outside the submitted work. U. Anido reports personal fees and nonfinancial support from Ipsen; personal fees from Bristol Myers Squibb, Eisai, MSD, and Merck & Co., Inc., Rahway, NJ; and grants and personal fees from Bayer outside the submitted work. O. Huillard reports personal fees from Advanced Accelerator Applications, Bayer, Sanofi, MSD, Bristol Myers Squibb, Ipsen, Pfizer, Eisai, AstraZeneca, Novartis, and Janssen outside the submitted work. J. Ma is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ. M. Rajasagi was an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, at the time of this analysis. A. Vajdi is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, and owns stock in Merck & Co., Inc., Rahway, NJ. J. Lunceford is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, and owns stock in Merck & Co., Inc., Rahway, NJ. R. Cristescu is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, and owns stock in Merck & Co., Inc., Rahway, NJ. K. Imai is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, and owns stock in Merck & Co., Inc., Rahway, NJ. B. Homet Moreno is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, and owns stock in Merck & Co., Inc., Rahway, NJ. N. Matsubara reports grants from MSD during the conduct of the study, as well as personal fees from Sanofi and grants from AbbVie, Amgen, Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Chugai Pharma, Eisai, Janssen, Eli Lilly and Company, Loxo Oncology, Novartis, Bayer, Pfizer, Roche, Seagen, Taiho, and Takeda outside the submitted work. No disclosures were reported by the other authors.
Figures


References
-
- Miyazaki J, Nishiyama H. Epidemiology of urothelial carcinoma. Int J Urol 2017;24:730–4. - PubMed
-
- Powles T, Bellmunt J, Comperat E, De Santis M, Huddart R, Loriot Y, et al. . Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:244–58. - PubMed
-
- van der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, et al. . Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N Engl J Med 2023;389:1778–89. - PubMed
-
- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, et al. . Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med 2024;390:875–88. - PubMed
-
- Galsky MD, Banchereau R, Hamidi HR, Leng N, Harris W, O’Donnell PH, et al. . Tumor, immune, and stromal characteristics associated with clinical outcomes with atezolizumab (atezo) + platinum-based chemotherapy (PBC) or atezo monotherapy (mono) versus PBC in metastatic urothelial cancer (mUC) from the phase III IMvigor130 study. J Clin Oncol 2020;38(15_suppl):5011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

