Noninvasive modulation of the hippocampal-entorhinal complex during spatial navigation in humans
- PMID: 39475597
- PMCID: PMC11524170
- DOI: 10.1126/sciadv.ado4103
Noninvasive modulation of the hippocampal-entorhinal complex during spatial navigation in humans
Abstract
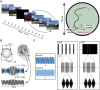
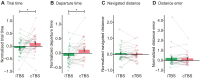
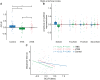
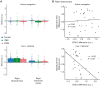
Because of the depth of the hippocampal-entorhinal complex (HC-EC) in the brain, understanding of its role in spatial navigation via neuromodulation was limited in humans. Here, we aimed to better elucidate this relationship in healthy volunteers, using transcranial temporal interference electric stimulation (tTIS), a noninvasive technique allowing to selectively neuromodulate deep brain structures. We applied tTIS to the right HC-EC in either continuous or intermittent theta-burst stimulation patterns (cTBS or iTBS), compared to a control condition, during a virtual reality-based spatial navigation task and concomitant functional magnetic resonance imaging. iTBS improved spatial navigation performance, correlated with hippocampal activity modulation, and decreased grid cell-like activity in EC. Collectively, these data provide the evidence that human HC-EC activity can be directly and noninvasively modulated leading to changes of spatial navigation behavior. These findings suggest promising perspectives for patients suffering from cognitive impairment such as following traumatic brain injury or dementia.
Figures


References
-
- Moffat S. D., Aging and spatial navigation: What do we know and where do we go? Neuropsychol. Rev. 19, 478–489 (2009). - PubMed
-
- Li A. W. Y., King J., Spatial memory and navigation in ageing: A systematic review of MRI and fMRI studies in healthy participants. Neurosci. Biobehav. Rev. 103, 33–49 (2019). - PubMed
-
- Head D., Isom M., Age effects on wayfinding and route learning skills. Behav. Brain Res. 209, 49–58 (2010). - PubMed
-
- Plácido J., de Almeida C. A. B., Ferreira J. V., de Oliveira Silva F., Monteiro-Junior R. S., Tangen G. G., Laks J., Deslandes A. C., Spatial navigation in older adults with mild cognitive impairment and dementia: A systematic review and meta-analysis. Exp. Gerontol. 165, 111852 (2022). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

