A genetically encoded actuator boosts L-type calcium channel function in diverse physiological settings
- PMID: 39475605
- PMCID: PMC11524184
- DOI: 10.1126/sciadv.adq3374
A genetically encoded actuator boosts L-type calcium channel function in diverse physiological settings
Abstract
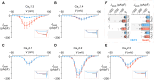
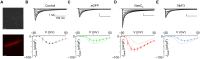
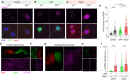
L-type Ca2+ channels (CaV1.2/1.3) convey influx of calcium ions that orchestrate a bevy of biological responses including muscle contraction, neuronal function, and gene transcription. Deficits in CaV1 function play a vital role in cardiac and neurodevelopmental disorders. Here, we develop a genetically encoded enhancer of CaV1.2/1.3 channels (GeeCL) to manipulate Ca2+ entry in distinct physiological settings. We functionalized a nanobody that targets the CaV complex by attaching a minimal effector domain from an endogenous CaV modulator-leucine-rich repeat containing protein 10 (Lrrc10). In cardiomyocytes, GeeCL selectively increased L-type current amplitude. In neurons in vitro and in vivo, GeeCL augmented excitation-transcription (E-T) coupling. In all, GeeCL represents a powerful strategy to boost CaV1.2/1.3 function and lays the groundwork to illuminate insights on neuronal and cardiac physiology and disease.
Figures



Update of
-
A Genetically Encoded Actuator Selectively Boosts L-type Calcium Channels in Diverse Physiological Settings.bioRxiv [Preprint]. 2023 Sep 23:2023.09.22.558856. doi: 10.1101/2023.09.22.558856. bioRxiv. 2023. Update in: Sci Adv. 2024 Nov;10(44):eadq3374. doi: 10.1126/sciadv.adq3374. PMID: 37790372 Free PMC article. Updated. Preprint.
References
-
- B. Hille, Ionic channels of excitable membranes (Sinauer Associates, ed. 3, 2001), pp. 814.
-
- Hofmann F., Flockerzi V., Kahl S., Wegener J. W., L-type CaV1.2 calcium channels: From in vitro findings to in vivo function. Physiol. Rev. 94, 303–326 (2014). - PubMed
-
- Bers D. M., Cardiac excitation-contraction coupling. Nature 415, 198–205 (2002). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

