Measurement of α-synuclein as protein cargo in plasma extracellular vesicles
- PMID: 39475636
- PMCID: PMC11551346
- DOI: 10.1073/pnas.2408949121
Measurement of α-synuclein as protein cargo in plasma extracellular vesicles
Erratum in
-
Correction for Gilboa et al., Measurement of α-synuclein as protein cargo in plasma extracellular vesicles.Proc Natl Acad Sci U S A. 2025 Feb 11;122(6):e2426543122. doi: 10.1073/pnas.2426543122. Epub 2025 Jan 16. Proc Natl Acad Sci U S A. 2025. PMID: 39819088 Free PMC article. No abstract available.
-
Correction for Gilboa et al., Measurement of α-synuclein as protein cargo in plasma extracellular vesicles.Proc Natl Acad Sci U S A. 2025 Oct 7;122(40):e2522569122. doi: 10.1073/pnas.2522569122. Epub 2025 Oct 1. Proc Natl Acad Sci U S A. 2025. PMID: 41032528 Free PMC article. No abstract available.
Abstract
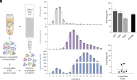
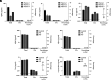
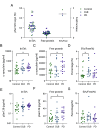
Extracellular vesicles (EVs) are released by all cells and hold great promise as a class of biomarkers. This promise has led to increased interest in measuring EV proteins from both total EVs as well as brain-derived EVs in plasma. However, measuring cargo proteins in EVs has been challenging because EVs are present at low levels, and EV isolation methods are imperfect at separating EVs from free proteins. Thus, knowing whether a protein measured after EV isolation is truly inside EVs is difficult. In this study, we developed methods to measure whether a protein is inside EVs and quantify the ratio of a protein in EVs relative to total plasma. To achieve this, we combined a high-yield size-exclusion chromatography protocol with an optimized protease protection assay and Single Molecule Array (Simoa) digital enzyme-linked immunoassays (ELISAs) for ultrasensitive measurement of proteins inside EVs. We applied these methods to analyze α-synuclein and confirmed that a small fraction of the total plasma α-synuclein is inside EVs. Additionally, we developed a highly sensitive Simoa assay for phosphorylated α-synuclein (phosphorylated at the Ser129 residue). We found enrichment in the phosphorylated α-synuclein to total α-synuclein ratio inside EVs relative to outside EVs. Finally, we applied the methods we developed to measure total and phosphorylated α-synuclein inside EVs from Parkinson's disease and Lewy body dementia patient samples. This work provides a framework for determining the levels of proteins in EVs and represents an important step in the development of EV diagnostics for diseases of the brain, as well as other organs.
Keywords: Parkinson’s Disease; alpha synuclein; biomarker; exosomes; extracellular vesicles.
Conflict of interest statement
Competing interests statement:D.R.W. is a founder, member of the Board of Directors, and equity holder in Quanterix. His interests were reviewed and are managed by BWH and Partners HealthCare in accordance with their conflict of interest policies. G.M.C. Disclosures: https://arep.med.harvard.edu/gmc/tech.html. The authors have filed IP on methods for EV isolation and analysis.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

