Hyponatremia unleashes neutrophil extracellular traps elevating life-threatening pulmonary embolism risk
- PMID: 39475645
- PMCID: PMC11551416
- DOI: 10.1073/pnas.2404947121
Hyponatremia unleashes neutrophil extracellular traps elevating life-threatening pulmonary embolism risk
Abstract
Neutrophil extracellular traps (NETs), essential for controlling infections, can induce various pathologies when dysregulated. Known triggers for infection-independent NETs release exist, yet a comprehensive understanding of the conditions prompting such responses is lacking. In this study, we identify hyponatremia as an independent inducer of NETs release, a common clinical condition that disrupts sodium/calcium exchange within neutrophils. This disruption leads to an excess of intracellular calcium, subsequent elevation of reactive oxygen species (ROS), and the citrullination of histone H3, culminating in the activation of NETs-release pathways. Notably, under hyponatremic conditions, this mechanism is exacerbated during infectious states, leading to the deposition of NETs in the lungs and increasing the risk of life-threatening pulmonary embolism. Our findings underscore the critical role of sodium and calcium homeostasis in neutrophil functionality and provide insights into the pathogenesis of hyponatremia-associated diseases, highlighting potential therapeutic interventions targeting NETs dynamics.
Keywords: SARS-CoV-2; hyponatremia; neutrophil extracellular traps; pulmonary embolism; sodium–calcium exchanger.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
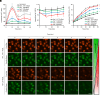
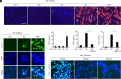
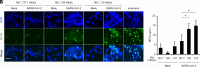
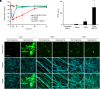
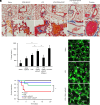
References
-
- Kolaczkowska E., Kubes P., Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013). - PubMed
-
- Brinkmann V., et al. , Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004). - PubMed
-
- Pilsczek F. H., et al. , A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 185, 7413–7425 (2010). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

