The zinc transporter Slc30a1 (ZnT1) in macrophages plays a protective role against attenuated Salmonella
- PMID: 39475776
- PMCID: PMC11524588
- DOI: 10.7554/eLife.89509
The zinc transporter Slc30a1 (ZnT1) in macrophages plays a protective role against attenuated Salmonella
Abstract
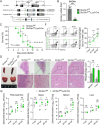
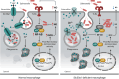
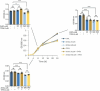
The zinc transporter Slc30a1 plays an essential role in maintaining cellular zinc homeostasis. Despite this, its functional role in macrophages remains largely unknown. Here, we examine the function of Slc30a1 in host defense using mice models infected with an attenuated stain of Salmonella enterica Typhimurium and primary macrophages infected with the attenuated Salmonella. Bulk transcriptome sequencing in primary macrophages identifies Slc30a1 as a candidate in response to Salmonella infection. Whole-mount immunofluorescence and confocal microscopy imaging of primary macrophage and spleen from Salmonella-infected Slc30a1flag-EGFP mice demonstrate Slc30a1 expression is increased in infected macrophages with localization at the plasma membrane and in the cytosol. Lyz2-Cre-driven Slc30a1 conditional knockout mice (Slc30a1fl/fl;Lyz2-Cre) exhibit increased susceptibility to Salmonella infection compared to control littermates. We demonstrate that Slc30a1-deficient macrophages are defective in intracellular killing, which correlated with reduced activation of nuclear factor kappa B and reduction in nitric oxide (NO) production. Notably, the model exhibits intracellular zinc accumulation, demonstrating that Slc30a1 is required for zinc export. We thus conclude that zinc export enables the efficient NO-mediated antibacterial activity of macrophages to control invading Salmonella.
Keywords: Salmonella infection; Slc30a1; ZnT1; iNOS; macrophages; medicine; mouse; zinc accumulation.
© 2024, Na-Phatthalung et al.
Conflict of interest statement
PN, SS, EX, JW, JM, FW No competing interests declared
Figures



















Update of
- doi: 10.1101/2023.06.06.543958
References
-
- Botella H, Peyron P, Levillain F, Poincloux R, Poquet Y, Brandli I, Wang C, Tailleux L, Tilleul S, Charrière GM, Waddell SJ, Foti M, Lugo-Villarino G, Gao Q, Maridonneau-Parini I, Butcher PD, Castagnoli PR, Gicquel B, de Chastellier C, Neyrolles O. Mycobacterial p1-type atpases mediate resistance to zinc poisoning in human macrophages. Cell Host & Microbe. 2011;10:248–259. doi: 10.1016/j.chom.2011.08.006. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

