Evaluating the Impact of a Game (Inner Dragon) on User Engagement Within a Leading Smartphone App for Smoking Cessation: Randomized Controlled Trial
- PMID: 39475840
- PMCID: PMC11561441
- DOI: 10.2196/57839
Evaluating the Impact of a Game (Inner Dragon) on User Engagement Within a Leading Smartphone App for Smoking Cessation: Randomized Controlled Trial
Abstract
Background: Smartphone apps are a convenient, low-cost approach to delivering smoking cessation support to large numbers of individuals. Yet, the apps are susceptible to low rates of user engagement and retention.
Objective: This study aims to test the effects of a new game module (called Inner Dragon) integrated into Smoke Free (23 Limited), a leading smoking cessation app with established efficacy. The primary outcomes measured user engagement with the app.
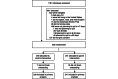
Methods: A 2-arm, parallel-group, randomized controlled trial was conducted in the United States with an 8-week follow-up. Adult individuals who smoked ≥1 cigarettes daily and planned to quit smoking within 7 days were recruited and randomized (N=500), with equal allocation. Both groups received free access to the original Smoke Free app with "core" features of its smoking cessation program (eg, a diary and craving log). The treated group received additional access to the integrated Inner Dragon game that incorporated several game mechanics designed to increase user engagement. User engagement outcomes were the number of unique app sessions, average minutes per session, days with a session, and program adherence. Self-reported and verified smoking abstinence and app satisfaction were also assessed. The main analysis estimated the intention-to-treat effect of access to Inner Dragon on each outcome. Further analyses assessed effect modification by participant characteristics and the association of intensity of game use with program adherence and abstinence.
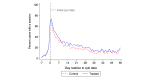
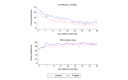
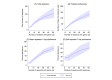
Results: Overall, user engagement was greater for treated versus control participants: they had 5.3 more sessions of Smoke Free (mean 29.6, SD 36.5 sessions vs mean 24.3, SD 37.9 sessions; P=.06), 0.8 more minutes per session (mean 6.9, SD 5.4 min vs mean 6.1, SD 5.2 min; P=.047), and 3.4 more days with a session (mean 14.3, SD 15.3 days vs mean 11.9, SD 14.3 days; P=.03). Program adherence, based on the number of times core features of the original Smoke Free app were used, was higher for treated versus control participants (mean 29.4, SD 41.3 times vs mean 22.6, SD 35.6 times; P=.03). Self-reported 7-day and 30-day point-prevalence abstinence and verified 7-day point-prevalence abstinence at 8 weeks did not significantly differ by study group. The mean repeated 1-day prevalence of quitting was higher among the treated group versus the control group (mean 17.3%, SD 25.6 vs mean 12.4%, SD 21.3; P=.01). App satisfaction and the motivation to (stay) quit did not differ by study group. Higher intensity of game use was associated with increased program adherence and self-reported abstinence.
Conclusions: Findings suggest that the Inner Dragon game increased user engagement and program adherence. Additional refinements to the game design may clarify whether the game increases abstinence rates. Overall, it is feasible to deploy games and gamification to enhance user engagement in existing smoking cessation interventions.
Trial registration: ClinicalTrials.gov NCT05227027; https://clinicaltrials.gov/study/NCT05227027.
Keywords: engagement; games for health; gamification; mobile app; mobile phone; randomized controlled trial; smoking cessation.
©Justin S White, Séverine Toussaert, Bethany R Raiff, Marie K Salem, Amy Yunyu Chiang, David Crane, Edward Warrender, Courtney R Lyles, Lorien C Abroms, J Lee Westmaas, Johannes Thrul. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 30.10.2024.
Conflict of interest statement
Conflicts of Interest: BRR is the founder of Re-Connect Health, Inc, a company that develops mobile apps for health behavior change. CRL was a visiting researcher at Google LLC during the preparation of this manuscript. DC is the founder and chief executive officer and EW is the chief technology officer of the Smoke Free app, and both derive income from it. LCA receives royalties for the sale of Text2Quit, a quit smoking program. JT reports membership on the scientific advisory board of MindCotine, Inc, which offers a smoking cessation program. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflicts of interest policies. All other authors declare no other conflicts of interest.
Figures
References
-
- US Department of Health and Human Services . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; 2014.
-
- U.S. National Cancer Institute and World Health Organization The economics of tobacco and tobacco control. National Cancer Institute. 2016. [2023-10-01]. https://cancercontrol.cancer.gov/brp/tcrb/monographs/monograph-21 .
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

