Characterization of patients with Duchenne muscular dystrophy across previously developed health states
- PMID: 39475941
- PMCID: PMC11524485
- DOI: 10.1371/journal.pone.0307118
Characterization of patients with Duchenne muscular dystrophy across previously developed health states
Abstract
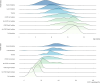
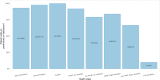
Project HERCULES has developed a natural history model (NHM) of disease progression in Duchenne muscular dystrophy (DMD) that comprises eight ordered health states (two ambulatory states, one transfer state indicating increased caregiver burden in which patients cannot walk/run 10m or rise from floor but can still support their own weight, and five non-ambulatory states). The current study used data from nine sources (clinical trial placebo arms, one real-world dataset, and three natural history datasets) to further characterize patients with DMD according to these health states. The study included 1,173 patients across 5,306 visits. Patients were on average older and exhibited worse ambulatory, pulmonary, upper limb, and cardiac functions with each successive health state. Mean±SE ages increased monotonically across health states, starting with 8.47±0.07 for early ambulatory, 10.86±0.13 for late ambulatory, 11.65±0.35 for transfer state, and ranging from 13.17±0.32 to 16.84±0.37 for the non-ambulatory states. North Star Ambulatory Assessment (NSAA) total score, which measures motor function and ranges from 34 (best) to 0 (worst), was 23.7 (interquartile range [IQR]: 20-30) for early ambulatory patients, 12.7 (IQR: 9-16) for late ambulatory patients, and 3.9 (IQR: 2-5) for transfer patients. Pulmonary function as measured by mean±SE of forced vital capacity percent predicted (FVC%p) was 94.5±0.8 for early ambulatory, 89.1±1.4 for late ambulatory, and 80.2±2.8 for transfer states, and decreased from 77.2±1.7 to 20.6±1.6 across the five non-ambulatory health states. In summary, these findings further characterize health states and their interpretation in economic modeling and decision-making in DMD management.
Copyright: © 2024 Muntoni et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: FM is a member of the Rare Disease Scientific Advisory Group for Pfizer and of Dyne Therapeutics SAB and has participated in SAB meetings for PTC, Sarepta, Pfizer, Roche, Santhera, and Wave Therapeutics. UCL and Great Ormond Street Hospital have received funding from Pfizer, Italfarmaco, Wave, Santhera, Roche, NF Pharma, ReveraGen, Genethon, and Sarepta regarding clinical trials. NG has received compensation for consultancy services from Eli Lilly, Italfarmaco, PTC Therapeutics, BioMarin Pharmaceutical, Pfizer, Avidity, Daiichi Sankyo, Wave, and Santhera and has served as site investigator for GlaxoSmithKline, Prosensa, BioMarin Pharmaceutical, Italfarmaco, Eli Lilly, Wave, and Sarepta. NP and JA are employees of Pfizer Inc. and own stock/stock options. JS cofounded the cTAP and is an employee of Analysis Group, Inc., a consulting firm that received funding from the membership of cTAP to conduct this study. MJ is an employee of Analysis Group, Inc., a consulting firm that received funding from the membership of cTAP to conduct this study. SJW cofounded and manages cTAP and has received funding from the membership of cTAP to facilitate this study. CMM has served as a consultant for PTC Therapeutics, BioMarin Pharmaceutical, Sarepta Therapeutics, Eli Lilly, Pfizer Inc., Santhera Pharmaceuticals, Cardero Therapeutics, Inc., Catabasis Pharmaceuticals, Capricor Therapeutics, Astellas Pharma (Mitobridge), and FibroGen, Inc.; serves on external advisory boards related to DMD for PTC Therapeutics, Sarepta Therapeutics, Santhera Pharmaceuticals, and Capricor Therapeutics; and reports grants from the US Department of Education/National Institute on Disability and Rehabilitation Research, National Institute on Disability, Independent Living, and Rehabilitation Research, US NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH/National Institute of Neurologic Disorders and Stroke, US Department of Defense, and Parent Project Muscular Dystrophy US. EM has served on clinical steering committees and/or as a consultant for Eli Lilly, Italfarmaco, PTC Therapeutics, Sarepta, Santhera, and Pfizer; has served as PI for GlaxoSmithKline, Prosensa, BioMarin Pharmaceutical, Italfarmaco, Roche, PTC, Pfizer, Sarepta, Santhera, Wave, NS Pharma, and Eli Lilly. PD, KB, and MM were Pfizer Inc. employees at the time the study was conducted. CH was an Analysis Group, Inc. employee at the time the study was conducted. This does not alter our adherence to PLoS One policies on sharing data and materials.
Figures


References
-
- Crisafulli S, Sultana J, Fontana A, Salvo F, Messina S, Trifirò G. Global epidemiology of Duchenne muscular dystrophy: an updated systematic review and meta-analysis. Orphanet J Rare Dis. 2020;15(1):141. Epub 20200605. doi: 10.1186/s13023-020-01430-8 ; PubMed Central PMCID: PMC7275323. - DOI - PMC - PubMed
-
- Salari N, Fatahi B, Valipour E, Kazeminia M, Fatahian R, Kiaei A, et al.. Global prevalence of Duchenne and Becker muscular dystrophy: a systematic review and meta-analysis. J Orthop Surg Res. 2022;17(1):96. Epub 20220215. doi: 10.1186/s13018-022-02996-8 ; PubMed Central PMCID: PMC8848641. - DOI - PMC - PubMed
-
- Landfeldt E, Thompson R, Sejersen T, McMillan HJ, Kirschner J, Lochmüller H. Life expectancy at birth in Duchenne muscular dystrophy: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35(7):643–53. Epub 20200227. doi: 10.1007/s10654-020-00613-8 ; PubMed Central PMCID: PMC7387367. - DOI - PMC - PubMed

