Slowing Cognitive Decline in Major Depressive Disorder and Mild Cognitive Impairment: A Randomized Clinical Trial
- PMID: 39476073
- PMCID: PMC11525663
- DOI: 10.1001/jamapsychiatry.2024.3241
Slowing Cognitive Decline in Major Depressive Disorder and Mild Cognitive Impairment: A Randomized Clinical Trial
Abstract
Importance: Older adults with major depressive disorder (MDD) or mild cognitive impairment (MCI) are at high risk for cognitive decline.
Objective: To assess the efficacy of cognitive remediation (CR) plus transcranial direct current stimulation (tDCS) targeting the prefrontal cortex in slowing cognitive decline, acutely improving cognition, and reducing progression to MCI or dementia in older adults with remitted MDD (rMDD), MCI, or both.
Design, setting, and participants: This randomized clinical trial was conducted at 5 academic hospitals in Toronto, Ontario, Canada. Participants were older adults who had rMDD (with or without MCI, age ≥65 y) or MCI without rMDD (age ≥60 y). Assessments were made at baseline, month 2, and yearly from baseline for 3 to 7 years.
Interventions: CR plus tDCS (hereafter, active) or sham plus sham 5 days a week for 8 weeks followed by twice-a-year 5-day boosters and daily at-home CR or sham CR.
Main outcomes and measures: The primary outcome was change in global composite cognitive score. Secondary outcomes included changes in 6 cognitive domains, moderating effect of the diagnosis, moderating effect of APOE ε4 status, change in composite score at month 2, and progression to MCI or dementia over time.
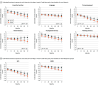
Results: Of 486 older adults who provided consent, 375 (with rMDD, MCI, or both) received at least 1 intervention session (mean [SD] age, 72.2 [6.4] years; 232 women [62%] and 143 men [38%]). Over a median follow-up of 48.3 months (range, 2.1-85.9), CR and tDCS slowed cognitive decline in older adults with rMDD or MCI (adjusted z score difference [active - sham] at month 60, 0.21; 95% CI, 0.07 to 0.35; likelihood ratio test [LRT] P = .006). In the preplanned primary analysis, CR and tDCS did not improve cognition acutely (adjusted z score difference [active - sham] at month 2, 0.06, 95% CI, -0.006 to 0.12). Similarly, the effect of CR and tDCS on delaying progression from normal cognition to MCI or MCI to dementia was weak and not significant (hazard ratio, 0.66; 95% CI, 0.40 to 1.08; P = .10). Preplanned analyses showed treatment effects for executive function (LRT P = .04) and verbal memory (LRT P = .02) and interactions with diagnosis (P = .01) and APOE ε4 (P < .001) demonstrating a larger effect among those with rMDD and in noncarriers of APOE ε4.
Conclusions and relevance: The study showed that CR and tDCS, both targeting the prefrontal cortex, is efficacious in slowing cognitive decline in older adults at risk of cognitive decline, particularly those with rMDD (with or without MCI) and in those at low genetic risk for Alzheimer disease.
Trial registration: ClinicalTrials.gov Identifier: NCT02386670.
Conflict of interest statement
Figures


References
-
- Reynolds CF III, Butters MA, Lopez O, et al. Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch Gen Psychiatry. 2011;68(1):51-60. doi: 10.1001/archgenpsychiatry.2010.184 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

