The Functional Transcriptomic Landscape Informs Therapeutic Strategies in Multiple Myeloma
- PMID: 39476082
- PMCID: PMC11733535
- DOI: 10.1158/0008-5472.CAN-24-0886
The Functional Transcriptomic Landscape Informs Therapeutic Strategies in Multiple Myeloma
Abstract
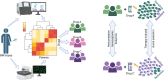
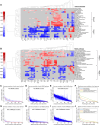
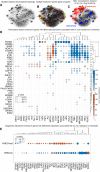
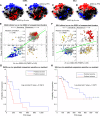
Several therapeutic agents have been approved for treating multiple myeloma, a cancer of bone marrow-resident plasma cells. Predictive biomarkers for drug response could help guide clinical strategies to optimize outcomes. In this study, we present an integrated functional genomic analysis of tumor samples from patients multiple myeloma that were assessed for their ex vivo drug sensitivity to 37 drugs, clinical variables, cytogenetics, mutational profiles, and transcriptomes. This analysis revealed a multiple myeloma transcriptomic topology that generates "footprints" in association with ex vivo drug sensitivity that have both predictive and mechanistic applications. Validation of the transcriptomic footprints for the anti-CD38 mAb daratumumab (DARA) and the nuclear export inhibitor selinexor (SELI) demonstrated that these footprints can accurately classify clinical responses. The analysis further revealed that DARA and SELI have anticorrelated mechanisms of resistance, and treatment with a SELI-based regimen immediately after a DARA-containing regimen was associated with improved survival in three independent clinical trials, supporting an evolutionary-based strategy involving sequential therapy. These findings suggest that this unique repository and computational framework can be leveraged to inform underlying biology and to identify therapeutic strategies to improve treatment of multiple myeloma. Significance: Functional genomic analysis of primary multiple myeloma samples elucidated predictive biomarkers for drugs and molecular pathways mediating therapeutic response, which revealed a rationale for sequential therapy to maximize patient outcomes.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
P.R. Sudalagunta reports personal fees from FORUS Therapeutics Inc. outside the submitted work; in addition, P.R. Sudalagunta has a patent for “A model of clinical synergy in cancer, PCT/US2020/062232 (WO/2021/108551-A1)” pending, a patent for “A multiomic approach to modeling of gene regulatory networks in multiple myeloma, PCT/US2022/024217 (WO/2022/217136-A1)” pending, and a patent for “Altering epigenetic landscapes control progression and refractory disease states in multiple myeloma, PCT/US2023/078667 (WO/2024/097981)” pending. R.R. Canevarolo reports a patent for WO2024097981A1 pending to Moffitt Cancer Center, a patent for WO2022217136A1 pending to Moffitt Cancer Center, and a patent for WO2021108551A1 pending to Moffitt Cancer Center. M.B. Meads reports a patent for “A model of clinical synergy in cancer,” PCT/US2020/062232 (WO/2021/108551-A1), priority date November 25, 2019, pending to H. Lee Moffitt Cancer Center and Research Institute, a patent for “A multiomic approach to modeling of gene regulatory networks in multiple myeloma,” PCT/US2022/024217 (WO/2022/217136-A1), priority date October 04, 2021, pending to H. Lee Moffitt Cancer Center and Research Institute, and a patent for “Altering epigenetic landscapes control progression and refractory disease states in multiple myeloma,” PCT/US2023/078667 (WO/2024/09798-A1), priority date March 11, 2022, pending to H. Lee Moffitt Cancer Center and Research Institute. O. Hampton reports O. Hampton was a paid employee of Aster Insights while conduction of research. J.K. Teer reports grants from the NIH during the conduct of the study; in addition, J.K. Teer has a patent for Negative Information Storage Model issued. B.D. Shah reports grants, personal fees, and other support from Kite/Gilead, other support from Servier and Pepromene Bio, personal fees and other support from Jazz, and personal fees from Novartis, Deciphera, Takeda, Beigene, Pfizer, Bristol Myers Squibb, Amgen, Adaptive, Lilly/Loxo, from Autolus, and Syndax outside the submitted work. L. Hazlehurst reports being a cofounder of Modulation Therapeutics, but this work is not related to the current pipeline at Modulation Therapeutics. Y. Chai reports other support from Karyopharm Therapeutics during the conduct of the study. A. DeCastro reports was a former employee of one of the therapeutics tested in the study (Karyopharm Therapeutics). E.M. Siegel reports grants from the NIH NCI outside the submitted work. M. Alsina reports grants from Bristol Myers Squibb and other support from Janssen and Sanofi outside the submitted work. T. Nishihori reports other support from Novartis and Karyopharm Therapeutics outside the submitted work. J.L. Cleveland reports grants from the NCI/NIH during the conduct of the study. W. Dalton reports grants from Karyopharm Therapeutics during the conduct of the study and personal fees from AsterInsights outside the submitted work. C.J. Walker reports other support from Karyopharm Therapeutics during the conduct of the study. Y. Landesman used to work for Karyopharm Therapeutics and still holds stock of the company. R. Baz reports personal fees and other support from Janssen, other support from Abbvie, Regeneron, and Bristol Myers Squibb, and personal fees from Pfizer and Cellectar outside the submitted work. A.S. Silva reports a patent for A.S. Silva, K.H. Shain, P.R. Sudalagunta, R.R. Canevarolo, and M.B. Meads, “A model of clinical synergy in cancer,” PCT/US2020/062232 (WO/2021/108551-A1), priority date November 25, 2019, issued, a patent for A.S. Silva, K.H. Shain, P.R. Sudalagunta, R.R. Canevarolo, and M.B. Meads, “A multiomic approach to modeling of gene regulatory networks in multiple myeloma,” PCT/US2022/024217 (WO/2022/217136-A1), priority date October 04, 2021, pending, and a patent for A.S. Silva, K.H. Shain, P.R. Sudalagunta, R.R. Canevarolo, and M.B. Meads, “Altering epigenetic landscapes control progression and refractory disease states in multiple myeloma,” U.S. Provisional Application No. 63/422,106, priority date March 11, 2022, pending. K.H. Shain reports grants and personal fees from Karyopharm Therapeutics during the conduct of the study and grants from Abbvie and personal fees from Bristol Myers Squibb, Janssen, Amgen, Regeneron, Adaptive, and Sanofi outside the submitted work; in addition, K.H. Shain has a patent for 10110-243US1 pending and a patent for 10110-363WO1 issued. No disclosures were reported by the other authors.
Figures


References
-
- Moore DC, Oxencis CJ, Shank BR. New and emerging pharmacotherapies for management of multiple myeloma. Am J Health Syst Pharm 2022;79:1137–45. - PubMed
-
- Kumar SK, Callander NS, Adekola K, Anderson L, Baljevic M, Campagnaro E, et al. Multiple myeloma, version 3.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2020;18:1685–717. - PubMed
MeSH terms
Substances
Grants and funding
- Florida Department of Health (DOH)
- Multiple Myeloma Research Foundation (MMRF)
- U01 CA261841/CA/NCI NIH HHS/United States
- U54 CA193489/CA/NCI NIH HHS/United States
- R01 CA195727/CA/NCI NIH HHS/United States
- P30 CA076292/CA/NCI NIH HHS/United States
- R21 CA164322/CA/NCI NIH HHS/United States
- P30-CA076292/National Cancer Institute (NCI)
- R01 CA194051/CA/NCI NIH HHS/United States
- Pentecost Myeloma Research Center (PMRC)
- 1U54CA193489-01A1/National Cancer Institute (NCI)
- 1R01CA195727/National Cancer Institute (NCI)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

