Forimtamig, a novel GPRC5D-targeting T-cell bispecific antibody with a 2+1 format, for the treatment of multiple myeloma
- PMID: 39476124
- PMCID: PMC11738037
- DOI: 10.1182/blood.2024025987
Forimtamig, a novel GPRC5D-targeting T-cell bispecific antibody with a 2+1 format, for the treatment of multiple myeloma
Abstract
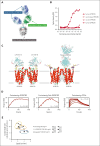
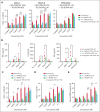
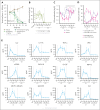
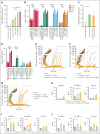
Despite several approved therapies, multiple myeloma (MM) remains an incurable disease with high unmet medical need. "Off-the-shelf" T-cell bispecific antibodies (TCBs) targeting B-cell maturation antigen (BCMA) and G protein-coupled receptor class C group 5 member D (GPRC5D) have demonstrated high objective response rates in heavily pretreated patients with MM; however, primary resistance, short duration of response, and relapse driven by antigen shift frequently occur. Although GPRC5D represents the most selective target in MM, recent findings indicate antigen loss occurs more frequently than with BCMA. Thus, anti-GPRC5D immunotherapies must hit hard during a short period of time. Here, we characterize forimtamig, a novel GPRC5D-targeting TCB with 2+1 format. Bivalent binding of forimtamig to GPRC5D confers higher affinity than classical 1+1 TCB formats correlating with formation of more stable immunological synapses and higher potency in tumor cell killing and T-cell activation. Using an orthotopic mouse model of MM, forimtamig recruited T effector cells to the bone marrow and induced rapid tumor killing even after the introduction of step-up dosing to mitigate cytokine release. Combination of forimtamig with standard-of-care agents including anti-CD38 antibodies, immunomodulatory drugs, and proteasome inhibitors improved depth and duration of response. The combination of forimtamig with novel therapeutic agents including BCMA TCB and cereblon E3 ligase modulatory drugs was potent and prevented occurrence of GPRC5D -negative tumor relapse. Forimtamig is currently being evaluated in phase 1 clinical trials in patients with relapsed and refractory MM for monotherapy and in combination treatments. This trial was registered at www.ClinicalTrials.gov as #NCT04557150.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.E., T.F., R.R., C.H., T.P., G.H., R.F., S.G., R.A., C. Kirstenpfad, L.K., S. Lechner, A.B., I.D., A.-M.B., J.A., I.L., F.C., S. Leclair, T.C., G.F., C.D., L.M., C.B., Q.D., F.O., F.B., C.G., S.D., T.K., M.K., M.B., S. Lechner, and P.U. are employees of Roche. C.K., A.C., M.M., L. Bernasconi, and W.X. are former employees of Roche. S.D., F.B., C. Klein, I.D., T.F., J.A., L.K., C.H., F.C., M.B., and M.M. hold stock options of F. Hoffmann-La Roche. J.E., T.F., M.B., S.L., T.C., A.C., A.B., A.-M.B., I.D., F.B., L.K., F.C., G.F., C.G., S.D., C. Kirstenpfad, T.K., C.H., M.M., L.B., S. Leclair, W.X., C. Klein, and P.U. declare patents with Roche. The remaining authors declare no competing financial interests.
Figures








References
-
- Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021;398(10297):314–324. - PubMed
-
- Munshi NC, Jr., Shah N, Anderson LD, Jr., et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021;384(8):705–716. - PubMed
-
- San-Miguel J, Dhakal B, Yong K, et al. Cilta-cel or standard care in lenalidomide-refractory multiple myeloma. N Engl J Med. 2023;389(4):335–347. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

