A phase 2, multicenter, clinical trial of CPX-351 in older patients with secondary or high-risk acute myeloid leukemia: PETHEMA-LAMVYX
- PMID: 39476204
- PMCID: PMC11694237
- DOI: 10.1002/cncr.35618
A phase 2, multicenter, clinical trial of CPX-351 in older patients with secondary or high-risk acute myeloid leukemia: PETHEMA-LAMVYX
Abstract
Background: LAMVYX was a multicenter, single-arm, phase 2 trial designed to validate the safety and efficacy of CPX-351 in patients aged 60-75 years with newly diagnosed, secondary acute myeloid leukemia and to generate evidence on key issues not addressed in the preceding regulatory pivotal trial.
Methods: The primary end point of the study was the complete remission (CR)/CR with incomplete hematologic recovery (CRi) rate after induction. Eligible patients were recommended to undergo allogeneic hematopoietic stem cell transplantation after the first consolidation cycle. Alternatively, patients could undergo up to six maintenance cycles with CPX-351.
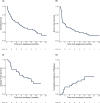
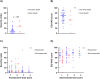
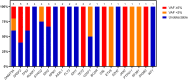
Results: Twenty-nine patients (49%; 95% exact confidence interval [CI], 37%-62%) patients achieved a CR/CRi after one or two cycles of induction, with a measurable residual disease negativity rate of 67% as assessed by centralized, multiparameter flow cytometry. Among patients who had serial next-generation sequencing analyses available, clearance of somatic mutations that were present at diagnosis was achieved in 7 (35%). The median follow-up among survivors was 16.8 months (range, 8.7-24.3 months). The median event-free survival was 3.0 months (95% CI, 1.4-7.3 months), and the median overall survival was 7.4 months (95% CI, 3.7-12.7 months). In landmark analyses at day +100 from diagnosis, the 1-year overall and event-free survival rate among patients who underwent allogeneic hematopoietic stem cell transplantation was 70% (95% CI, 47%-100%) and 70% (95% CI, 47%-100%), respectively. The corresponding values were 89% (95% CI, 71%-100%) and 44% (95% CI, 21%-92%), respectively, for patients who entered the maintenance phase. No significant longitudinal changes were observed in severity index or quality-of-life visual analog scale scores.
Conclusions: The current data provide novel insights that might inform the clinical positioning and optimal use of CPX-351, complementing previous results (ClinicalTrials.gov identifier NCT04230239).
Keywords: acute myeloid leukemia; allogeneic hematopoietic stem cell transplantation; maintenance therapy; measurable residual disease; quality of life.
© 2024 The Author(s). Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Eduardo Rodríguez‐Arbolí reports personal/consulting fees from Astellas Pharma, Laboratories Delbert, and Servier Pharmaceuticals; support for other professional activities from AbbVie and Astellas Pharma Europe; and travel support from AbbVie, Astellas Pharma, Eurocept, Gilead Sciences Inc., and Jazz Pharmaceuticals outside the submitted work. Juan M. Bergua reports travel support from F. Hoffman‐La Roche AG outside the submitted work. Susana Vives reports personal/consulting fees from Jazz Pharmaceuticals outside the submitted work. Teresa Bernal reports personal/consulting fees from AbbVie and Jazz Pharmaceuticals; and support for other professional activities from Astellas Pharma outside the submitted work. Mar Tormo reports personal/consulting fees from AbbVie and Sobi; support for other professional activities from Jazz Pharmaceuticals; and travel support from Bristol Myers Squibb Company and Jazz Pharmaceuticals outside the submitted work. David Martínez‐Cuadrón reports personal/consulting fees from Astellas Pharma, Laboratories Delbert, and Otsuka Pharmaceutical; support for other professional activities from Servier Pharmaceuticals; and travel support from Otuska Pharmaceutical, Pfizer, and Servier Pharmaceuticals outside the submitted work. José A. Pérez‐Simón reports personal/consulting fees from Jazz Pharmaceuticals; and support for other professional activities from Incyte Corporation, Novartis, and Sanofi outside the submitted work. Pau Montesinos reports personal/consulting fees from AbbVie, Celgene, and Servier Pharmaceuticals outside the submitted work. The remaining authors disclosed no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

