Cardiovascular Risks With SGLT2 Inhibitors in Clinical Practice Among Patients With Type 2 Diabetes
- PMID: 39476235
- PMCID: PMC11525605
- DOI: 10.1001/jamanetworkopen.2024.41765
Cardiovascular Risks With SGLT2 Inhibitors in Clinical Practice Among Patients With Type 2 Diabetes
Abstract
Importance: Cardiovascular disease (CVD) can be recurrent during type 2 diabetes (T2D) progression in this aging population. The effectiveness of sodium-glucose cotransporter 2 inhibitor (SGLT2i) therapy on total (ie, first and subsequent) CVD among patients with T2D in clinical practice remains uncertain.
Objective: To analyze the comparative association of SGLT2i vs dipeptidyl peptidase 4 inhibitor (DPP4i) therapy with total CVD among patients with T2D in clinical practice.
Design, setting, and participants: This retrospective cohort study used electronic medical records at the National Cheng Kung University Hospital, a leading medical center in Taiwan, from 2015 through 2021. Adult patients with T2D who initiated first use of the study drugs from 2016 through 2019, with up to 6 years of follow-up, were identified.
Main outcomes and measures: The primary outcomes included total composite CVD events and individual CVD subtypes (ie, atrial fibrillation, coronary heart disease, heart failure, stroke, myocardial infarction, and transient ischemic attack). A shared frailty model analysis was used to assess the association of treatment with repeat CVD events. Data from patients at high risk for CVD recurrence were further analyzed. Data were analyzed from September 1, 2022, to December 31, 2023.
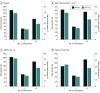
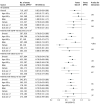
Results: Overall, 8384 patients with T2D were identified (mean [SD] age, 63.7 [12.4] years; 4645 [55.4%] male). A total of 1632 propensity score-matched pairs of SGLT2i (mean [SD] age, 57.8 [12.0] years; 673 [41.2%] female and 959 [58.8%] male) and DPP4i (mean [SD] age, 58.2 [12.9] years; 655 [40.1%] female and 977 [59.9%] male) users were included. SGLT2i was associated with reduced total CVD risk vs DPP4i therapy (hazard ratio [HR], 0.82 [95% CI, 0.69-0.98]) but not the first CVD event (with the use of SGLT2i therapy were more prominent for patients at high risk of CVD (ie, HR, 0.70 [95% CI, 0.62-0.80] for individuals with estimated glomerular filtration rate lower than 60 mL/min/1.73 m2; HR, 0.70 [95% CI, 0.64-0.78]; for individuals having any diabetes-related complications; and HR, 0.72 [95% CI, 0.65-0.80] for individuals with a history of CVD) compared with the overall cohort. Among patients at high risk of CVD, greater reduced total CVD burden associated with SGLT2i therapy was observed for women vs men (eg, HR, 0.59 [95% CI, 0.49-0.72] in the subgroup with CVD history).
Conclusions and relevance: In this cohort study of patients with T2D, the use of SGLT2is vs DPP4is was associated with reduced total cardiovascular burden, suggesting that long-term use of this therapy may optimize treatment benefit among patients with chronic CVD. The SGLT2i-associated benefit among patients with high risk of CVD encourages the prioritization of SGLT2i use for these vulnerable individuals.
Conflict of interest statement
Figures


References
-
- Mosenzon O, Alguwaihes A, Leon JLA, et al. ; CAPTURE Study Investigators . CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol. 2021;20(1):154. doi: 10.1186/s12933-021-01344-0 - DOI - PMC - PubMed
-
- Chen HY, Kuo S, Su PF, Wu JS, Ou HT. Health care costs associated with macrovascular, microvascular, and metabolic complications of type 2 diabetes across time: estimates from a population-based cohort of more than 0.8 million individuals with up to 15 years of follow-up. Diabetes Care. 2020;43(8):1732-1740. doi: 10.2337/dc20-0072 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

