Plasma exchange with albumin replacement for Alzheimer's disease treatment induced changes in serum and cerebrospinal fluid inflammatory mediator levels
- PMID: 39476248
- PMCID: PMC11651178
- DOI: 10.1002/acn3.52235
Plasma exchange with albumin replacement for Alzheimer's disease treatment induced changes in serum and cerebrospinal fluid inflammatory mediator levels
Abstract
Objective: There is extensive literature indicating that inflammatory pathways are affected in Alzheimer's disease (AD). We examined whether plasma exchange with albumin replacement (PE-Alb) can impact the inflammatory status of AD patients and alter the relationship between inflammatory mediators and cognitive measures.
Methods: Serum and cerebrospinal fluid (CSF) samples from 142 AD patients participating in the AMBAR trial (14-month schedule of PE-Alb treatment vs. placebo [sham PE-Alb]) were analyzed for changes from baseline for 19 inflammatory mediators (6 inflammatory cytokines, 9 chemokines, and 4 vascular injury indicators) at representative time points across the AMBAR study (lasting effects) as well as in pre- versus post-PE-Alb procedure (acute effects). Association between mediator changes and clinical outcomes reported in the AMBAR study (cognitive, functional, behavioral function, and global change tests) was assessed.
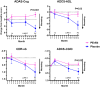
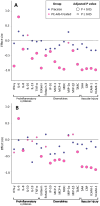
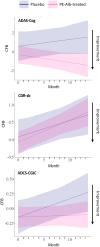
Results: PE-Alb significantly reduced IFN-γ, eotaxin, MIP-1α and ICAM-1 levels in serum, and eotaxin-3 and MIP-1β levels in CSF, at various time points during treatment (p < 0.05; false discovery rate-corrected). Vascular injury indicators were the mediators mostly affected by post- versus pre-PE-Alb level reduction. Increased serum MIP-1α levels were associated with worsening in ADAS-Cog, CDR-sb, and ADCS-CGIC scores in the placebo group, but not in the PE-Alb-treated group.
Interpretation: Peripheral intervention could affect AD by reducing inflammatory mediators in both peripheral and central compartments. Changes in MIP-1α due to PE-Alb were associated with changes in clinical outcomes.
© 2024 The Author(s). Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
RG, CM, MIB, and MC are full‐time employees of Grifols. AMO was a full‐time employee of Grifols when the study was performed. MB has received consultant fees from Araclon, Avid, Bayer, Elan, Grifols, Janssen/Pfizer, Lilly, Neuroptix, Nutricia, Roche, Sanofi, Biogen, and Servier; and received fees for lectures and funds for research from Araclon, Lilly, Grifols, Janssen, Novartis, Nutricia, Piramal, Pfizer‐Wyett, Roche, and Servier. OL has received consultant fees from Grifols and Lundbeck. AR has received been a consultant fees from Grifols, Prevail Therapeutics, and Landsteiner Genmed; reports grants/research funding from Abbvie, Janssen, Grifols, and Fundación Bancaria LaCaixa; and has stocks in Landsteiner Genmed.
Figures

References
-
- Cuberas‐Borrós G, Roca I, Castell‐Conesa J, et al. Neuroimaging analyses from a randomized, controlled study to evaluate plasma exchange with albumin replacement in mild‐to‐moderate Alzheimer's disease: additional results from the AMBAR study. Eur J Nucl Med Mol Imaging. 2022;49(13):4589‐4600. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
