Can Carbon be Used as an Anode for Water Splitting?
- PMID: 39476257
- PMCID: PMC11789973
- DOI: 10.1002/cssc.202401340
Can Carbon be Used as an Anode for Water Splitting?
Abstract
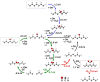
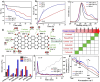
Carbon materials, whose structural and electronic properties can be fine-tuned, are promising material solutions for many energy-related systems. However, due to the lack of fundamental understanding of the carbon surface chemistry, especially when they are used in electrolytes, the rapid development of carbon as electrodes has led to many widely accepted misunderstandings. Focusing on the case of carbon-based electrode for water splitting, this Viewpoint tries to highlight the main problems of the area and demonstrates/presents the dynamic carbon surface chemistry in the application. The role of carbon as an anode for water splitting is revealed and if it can be practically used in water splitting is discussed.
Keywords: Anode; Carbon materials; Surface chemistry; Water splitting.
© 2024 The Authors. ChemSusChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lu X., He J., Huang L., Qin J., Ma Y., Liu X., Zhao W., Liu B., Zhang Z., Appl. Catal. B: Environ. 2023, 324, 122277.
-
- Lu S., Shi Y., Zhou W., Zhang Z., Wu F., Zhang B., J. Am. Chem. Soc. 2022, 144, 3250–3258. - PubMed
-
- Fan X., Zhou Y., Jin X., Song R. B., Li Z., Zhang Q., Carbon Energy 2021, 3, 449–472.
-
- Yang Z., Liu X., Ma X., Cao T., Xu J., Feng H., Diao R., Qi F., Huang H., Ma P., Adv. Funct. Mater. 2024, 34, 2310717.
-
- Xu H., Wang J., He H., Hwang I., Liu Y., Sun C., Zhang H., Li T., Muntean J. V., Xu T., Liu D.-J., J. Am. Chem. Soc. 2024, 146, 10357–10366. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

